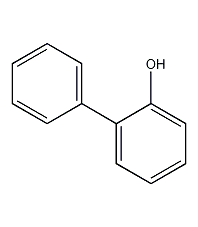
Structural formula
| Business number | 022C |
|---|---|
| Molecular formula | C12H10O |
| Molecular weight | 170.21 |
| label |
o-biphenol, o-phenylphenol, o-phenylphenol, o-Xenol, 2-Biphenol, 2-Hydroxybiphenol, Fungicide |
Numbering system
CAS number:90-43-7
MDL number:MFCD00002208
EINECS number:201-993-5
RTECS number:DV5775000
BRN number:606907
PubChem number:24869050
Physical property data
1. Properties: What precipitates from petroleum ether is white to light yellow or light pink needle-like crystals or crystalline powder, with an odor similar to phenol.
2. Density (g/mL, 25/4℃): 1.111
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 55.5~57.5
5. Boiling point (ºC, normal pressure): 286
6. Boiling point (ºC, 2.0265kPa): 152- 154
7. Refractive index: 1.528
8. Flash point (ºC, closed cup): 123
9. Specific rotation (º): Not available Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Hardly soluble in water, soluble in ethanol , acetone, isopropyl alcohol, ether, benzene and alkaline solution.
Toxicological data
Acute toxicity: Oral – rat LD50: 2000 mg/kg; Oral – mouse LC50: 1050 mg/kg.
Irritation data: Skin – Rabbit 20 mg/24 hours Moderate; Eyes – Rabbit 0.05 mg/24 hours Severe.
Ecological data
Irritating to skin and eyes. Staff should take precautions.
Molecular structure data
Molecular property data:
1. Molar refractive index: 52.72
2. Molar volume (cm3/mol): 153.1
3. Isotonic specific volume (90.2K): 395.6
4. Surface tension (dyne/cm): 44.5
5. Polarizability (10– 24cm3): 20.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecule polar surface area 20.2
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 149
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. White needle-like crystals or granular solids with a slight special odor.
2. Soluble in most organic solvents such as alcohol, ketone, benzene and sodium hydroxide solution, almost insoluble in water.
3. This product is non-toxic and odorless. It is a good preservative and can be used to prevent mildew and preserve fruits and vegetables.
4. Exists in flue-cured tobacco leaves and smoke.
Storage method
This product should be stored in a sealed, cool, dry place.
Synthesis method
1. (1) Cyclohexenylcyclohexanone is obtained by dehydrogenation in the presence of aluminum oxide; (2) 2-aminobiphenyl is reacted with sodium nitrite in sulfuric acid to obtain Diazotization produces biphenyl diazonium salt, which is then hydrolyzed.
2. Production of phenol by sulfonation method
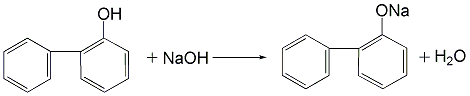
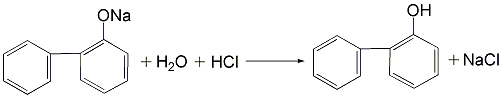
3.(1) Use the cyclohexanone route to prepare OPP, that is, using cyclohexanone as raw material, condensation and dehydration under acid catalysis to obtain the dimerization intermediate 2-(1-cyclohexenyl)cyclohexanone and 2-cyclohexylalkylenecyclohexanone are synthesized by dehydrogenation to o-phenylphenol. (2) Obtain a mixture of o-phenylphenol and p-phenylphenol from the by-product of phenol production by the sulfonation method, heat it and dissolve it in trichlorethylene, and precipitate para-phenylphenol crystals after cooling, and then centrifuge and filter. The solid is dried to obtain phenylphenol. After the mother liquor is washed with sodium carbonate solution, dilute sodium hydroxide is added to neutralize it, and then acidified to obtain o-phenylphenol.
4. Obtain o-phenylphenol and p-phenylphenol from the by-products of phenol production by sulfonation method The mixture is heated and dissolved in trichlorethylene. After cooling, p-phenylphenol crystals precipitate. After centrifugal filtration, the solid is dried to obtain p-phenylphenol. After the mother liquor is washed with sodium carbonate solution, dilute sodium hydroxide is added to neutralize it, and then acidified to obtain o-phenylphenol.
5. Tobacco: FC, 40.
Purpose
1. This product is used as an antifungal agent for plastics and rubber. It is also used as a carrier for carrier dyeing of hydrophobic synthetic fibers such as polyester and polyester, and as a heat stabilizer, surfactant, bactericidal preservative, and dye intermediate for plastics. This product and its sodium salt have high herbicidal activity, broad-spectrum sterilization and mildew removal capabilities, and are low-toxic and tasteless. They are good preservatives and can be used to prevent mildew and preserve fruits and vegetables, especially for citrus. It can also be used to treat lemons, pineapples, melons, fruits, pears, peaches, tomatoes, cucumbers, etc. to minimize rot. Countries such as the United Kingdom, the United States, and Canada are allowed to use a wider range of fruits, including apples. This product and its sodium salt can also be used as antiseptic and bactericidal agents in cosmetics, wood, leather, fiber and paper, etc. The general concentration is 0.15% to 1.5%. The U.S. Environmental Protection Agency (EPA) allows the use of nearly 200 varieties of antiseptic soaps, antiseptic and deodorant lotions, and antiseptic preservatives with o-phenylphenol or its sodium salt as the main ingredient, and considers such products to be non-toxic. , the annual consumption of o-phenylphenol in this field alone exceeds 1 million pounds (1 pound = 0.45359237kg).
2.Used in the manufacture of medicated soap.



 微信扫一扫打赏
微信扫一扫打赏
