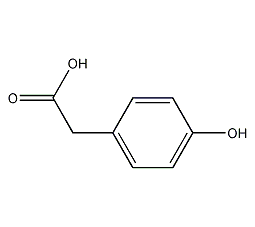
Structural formula
| Business number | 03ZK |
|---|---|
| Molecular formula | C9H8O3 |
| Molecular weight | 152 |
| label |
4-Hydroxyphenylacetic acid, 4-Hydroxyphenylacetic acid, 4-Hydroxybenzeneacetic acid, Pesticide intermediates; aromatic carboxylic acids and their derivatives, acidic solvent |
Numbering system
CAS number:156-38-7
MDL number:MFCD00004347
EINECS number:205-851-3
RTECS number:AI2680000
BRN number:1448766
PubChem ID:None
Physical property data
1. Properties: White crystalline powder.
2. Melting point (ºC): 149-150
3. Solubility: slightly soluble in water, soluble in ether, ethanol, ethyl acetate .
4. Boiling point (ºC): 346.6
5. Flash point (ºC): 177.6
6. Relative density (d20
sup>4) :1.319
Toxicological data
On skin: irritation to skin and mucous membranes
On eyes: irritating effects
Sensitization: No known sensitizing effects
Ecological data
None
Molecular structure data
1. Molar refractive index: 39.24
2. Molar volume (cm3/mol): 115.3
3. Isotonic specific volume (90.2K ): 320.6
4. Surface tension (dyne/cm): 59.8
5. Dielectric constant: not available
6. Dipole moment (10 -24cm3): Not available
7. Polarizability: 15.55
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 2
6. Topological molecule polar surface area 57.5
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 136
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Materials to avoid: Oxides. Hazardous decomposition products: carbon monoxide and carbon dioxide.
2. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
Storage method
Store in an airtight container and in a cool, dry place.
Synthesis method
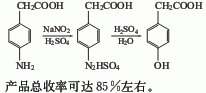
1.It is obtained by diazotization and hydrolysis of p-aminophenylacetic acid. Prepare sodium salt of p-aminophenylacetic acid and alkali solution, then add sulfuric acid. Cool to 0℃, control the temperature at 0-5℃, add sodium nitrate solution dropwise, and complete the reaction for 0.5h. . Add the obtained diazo liquid dropwise to the dilute sulfuric acid at 90-95°C, complete the dripping in about 1 hour, and then continue the reaction for 1 hour. The reaction solution is decolorized, filtered, cooled and extracted with ethyl acetate. The finished product is obtained after recovering ethyl acetate from the extract. The yield is about 85%.
2.3-Fluoro-p-hydroxyphenylacetic acid
2. Tobacco: OR, 41 ; FC, 54; BU, 26.
Purpose
Organic synthesis intermediate, used in the production of β-receptor blocker atenolol and the synthesis of 4,7-dihydroxyisoflavone, the active ingredient of kudzu daidzein; it can also be used as a pesticide intermediate.



 微信扫一扫打赏
微信扫一扫打赏
