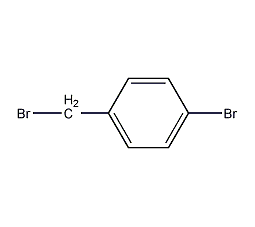
Structural formula
| Business number | 05ZV |
|---|---|
| Molecular formula | C7H6Br2 |
| Molecular weight | 249.93 |
| label |
4-Bromobenzyl bromide, p-bromobenzyl, 4-bromobenzyl, 4-bromobenzyl bromide, Alpha,4-dibromotoluene, p-bromobenzyl bromide, α,4-Dibromotoluene, BrC6H4CH2Br |
Numbering system
CAS number:589-15-1
MDL number:MFCD00000179
EINECS number:209-636-5
RTECS number:None
BRN number:606498
PubChem number:24850089
Physical property data
1. Properties: White needle-like crystals. Has a pleasant aromatic smell. Can evaporate with water vapor. Heating and sublimation.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 61
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 1.60kPa): 115~ 124
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturation Vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (% , V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ethanol, ether, carbon disulfide, Benzene and glacial acetic acid. Soluble in water and ethanol.
Toxicological data
None
Ecological data
None
Molecular structure data
None
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 75
10. Number of isotope atoms: 0
11. Determine the originalThe number of substereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. The number of determined chemical bond stereocenters: 0
14 .The number of uncertain chemical bond stereocenters: 0
15. The number of covalent bond units: 1
Properties and stability
1. Basic properties
Can evaporate with water vapor. Sublimation occurs when heated. Has an aromatic smell.
Storage method
None
Synthesis method
1. Add 1.4kg (8.3mol) p-bromotoluene and 10mL PCl3 to the flask and heat to 120℃ with stirring. Control the 115~125℃ and slowly add 1280g for about 3h. (430,8mol) bromonaphthalene, after adding, continue to keep stirring for 0.5h, let it stand and cool to below 10℃, the crystallization will be complete in 4~5h, pour off the uncrystallized part, melt the crystal, leave it at 20℃ overnight, and pour it off again The uncrystallized part was obtained, 0.94kg was distilled under reduced pressure, and the 120~124℃ (1.3~1.6kPa) fraction was collected to obtain 900g with a white appearance. The yield is about 45%. Dissolve 900g of the above steamed product in 700mL methanol, cool to room temperature (20°C) with stirring, filter out, rinse once with a little ice-cold methanol, and air-dry to obtain 810g solid with a purity of 99.2% (GC). 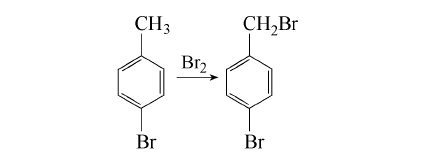
2. Preparation method:
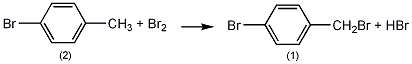
Pre-installed thermometer, reflux condenser (with calcium chloride on top In the reaction bottle of the drying tube (the drying tube is connected to the hydrogen bromide absorption device) and the dropping funnel, add 140g (0.83mol) of p-bromotoluene (2) and 3mL of PCl, heat to about 120°C with stirring, and slowly add bromine dropwise. 128g (0.8mol), control the temperature of the reaction solution between 115 and 125°C, and complete the addition in about 3 hours. After the addition was completed, the reaction was continued to stir for 0.5 h. Let cool to below 10°C and leave overnight. Decant off the uncrystallized liquid. Melt the crystals, place at 20°C overnight, and then pour off the uncrystallized liquid. Distill under reduced pressure and collect the fraction at 120~125℃/1.3kPa to obtain 89g of p-bromobenzyl bromide (1), with a yield of 45%. [1]
Purpose
Determination of aromatic carboxylic acids. Also used in other organic synthesis.



 微信扫一扫打赏
微信扫一扫打赏
