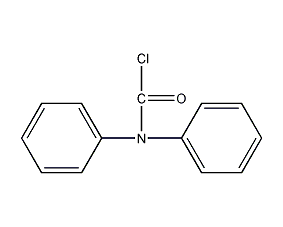
Structural formula
| Business number | 01T9 |
|---|---|
| Molecular formula | C13H10ClNO |
| Molecular weight | 231.68 |
| label |
Diphenylcarbamocarbon chloride, dimethylcarbamoyl chloride, N,N-Diphenylchloroformamide, diphenylcarbamide chloride, diphenylcarbamoyl chloride, Chloroformic acid diphenylamide, DPC-Cl |
Numbering system
CAS number:83-01-2
MDL number:MFCD00000633
EINECS number:201-450-2
RTECS number:EY5065000
BRN number:515312
PubChem number:24866819
Physical property data
1. Appearance: white powder
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air =1): Uncertain
4. Melting point (ºC): 85
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 5.2kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): Uncertain
9. Ratio Optical rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
p>
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC ): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Upper explosion limit (%, V/V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: Dissolved in most common solvents.
Toxicological data
None yet
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 65.21
2. Molar volume (cm3/mol): 182.5
3. Isotonic specific volume (90.2K): 487.2
4. Surface tension (dyne/cm): 50.8
5. Polarizability (10-24cm3): 25.85
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 3.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Topological molecular polar surface area (TPSA): 20.3
6. Number of heavy atoms: 16
7. Surface charge: 0
8. Complexity: 214
9. Number of isotope atoms: 0
10. Determine the number of atomic stereocenters : 0
11. Uncertain number of atomic stereocenters: 0
12. Determined number of chemical bond stereocenters: 0
13. Uncertain chemical bond formation Number of structural centers:0
14. Number of covalent bond units: 1
Properties and stability
It is hygroscopic and should be kept away from strong alkalis and strong oxidants; it is corrosive; unlike dimethylamino, diethylamino, and methylphenylcarbamoyl chloride, DPC-Cl does not have carcinogenic properties.
Storage method
Should be sealed and stored in a cool, dry place
Synthesis method
None yet
Purpose
1. Phenolic reagents. Organic Synthesis.
2. Diphenylcarbamoyl chloride (DPC-Cl) is a commonly used acylating reagent that can be used for Friedel-Crafts acylation; it can acylate amines, amino acids, thiols, phenols, and carboxylic acids Salt, etc.; can be used as a protective group during oligonucleotide synthesis, and the protective group can be removed under the action of concentrated ammonia-methanol or NaOH-methyl(ethanol) alcohol.
Introduction of carboxyl group Under the catalysis of AlCl3, DPC-Cl can introduce a carboxyl group on the aromatic ring. Alkyl or alkoxy substituted aromatic rings are susceptible to reaction. The product diphenylamide is hydrolyzed with alkali and then acidified to obtain carboxylic acid (formula 1)[1].
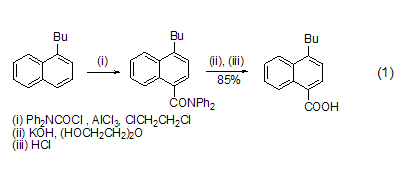
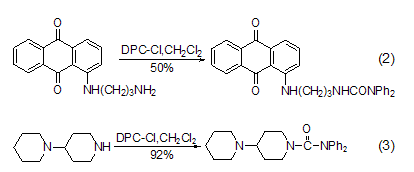
Reaction with amino groups DPC-Cl easily reacts with primary (formula 2) [2], secondary fatty amine (formula 3) [3] etc. [ 4~7].
Reaction with sulfhydryl group Under alkaline conditions (such as NaHCO3), DPC-Cl can react with thiols in ethanol solution (Formula 4)[8].
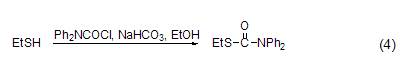
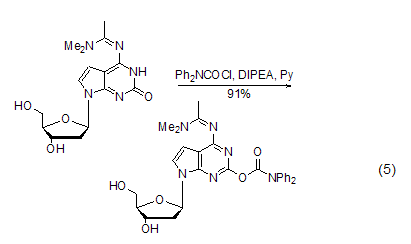
Used as oligomeric Protective groups during nucleotide synthesisWhen synthesizing oligonucleotides, the bases of guanine nucleosides are prone to side reactions and need to be protected. DPC-Cl can protect the enol isoforms of guanine bases. The conformation is protected by acylation. This method plays an important role in the synthesis of nucleoside compounds with guanine bases and those similar to guanine bases [9~12] ( Formula 5).
With double bonds Reaction of alcohol compounds DPC-Cl can undergo esterification reaction with alcohol compounds containing double bonds (Formula 6)[13].
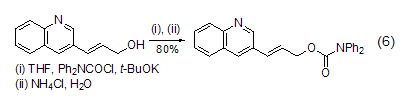
With azide Reaction of sodium DPC-Cl can react with sodium azide to generate the corresponding azide compound (Formula 7)[14].
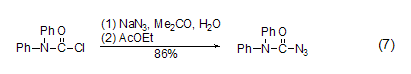
extended-reading:https://www.bdmaee.net/potassium-neodecanoate-2/extended-reading:https://www.bdmaee.net/n-dimethylaminopropyldiisopropanolamine/extended-reading:https://www.cyclohexylamine.net/dibutyltin-oxide-cas-818-08-6/extended-reading:https://www.bdmaee.net/polyurethane-reaction-inhibitor-y2300-polyurethane-reaction-inhibitor-reaction-inhibitor-y2300/extended-reading:https://www.bdmaee.net/dabco-k2097-catalyst-cas127-08-2-evonik-germany/extended-reading:https://www.newtopchem.com/archives/44959extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/TMR-4–TMR-4-trimer-catalyst-TMR-4.pdfextended-reading:https://www.bdmaee.net/dimethyltin-oxide/extended-reading:https://www.bdmaee.net/n-dimethylaminopropyl-diisopropanolamine-cas-63469-23-8-pc-cat-np10/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/33-11.jpg



 微信扫一扫打赏
微信扫一扫打赏
