1. The meaning of chelating resin
ChelationResinUsually refers to a functional resin with a cross-linked polymer as the backbone and special functional groups that can form complexes with metal ions through ionic bonds or coordination bonds. In most cases, it is composed of more than two The functional groups form cyclic compounds with metal ions. This functional resin usually has a hydrophobic skeleton and hydrophilic functional groups. Through the formation of electrostatic field, steric hindrance, synergy of functional groups, dilution or concentration and other polymer effects, a certain metal ion can be highly selectively complexed and adsorbed from a solution containing a variety of metal ions. The resin is insoluble and non-explosive, and can be recycled and used for enrichment, separation, analysis, recovery, etc. of metal ions.
Due to polymerchelateThe separation properties of the mixture have led to rapid development in applications in hydrometallurgy, inorganic chemistry, analytical chemistry, radiochemistry, marine chemistry, environmental chemistry, etc., such as treating industrial sewage and extracting from the ocean. Certain metals to make up for resource shortages, etc.

2. Classification of polymer chelating agents
(1) Classification according to the source of polymer chelating agents
This classification method can be roughly divided into natural polymer chelating agents and synthetic polymer chelating agents. The latter is obtained by synthetic methods according to people’s intentions, with large quantities and many varieties. There are also many natural polymer chelating agents in nature. Because there are many organic natural polymers in plants, animals, human bodies and other living organisms, such as cellulose, protein, peptides, alginic acid, chitin, etc. These natural polymers have various functional groups containing oxygen, nitrogen, sulfur, phosphorus, etc., such as hydroxyl, carboxyl, amineyl, amide group, phosphate group, etc., which can easily form coordination bonds with metal ions and generate natural polymer metal chelates. For example, heme complexed with iron atoms is the basic substance for the blood to transport oxygen in the human body; the blood of shrimp, which also has the function of transporting oxygen, is blue because its heme is complexed with copper atoms. It is an example of natural polymer chelating agents with similar structures complexing different metal atoms.
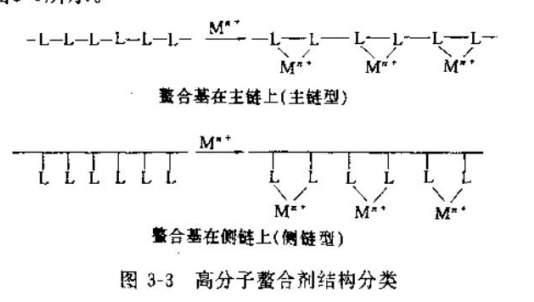
(2) Classification according to the structure of polymer chelating agents
Can be divided into two categories: one is the chelating group on the main chain, and the other is the chelating group is on the side chain. As shown in Figure 3-3
(3) According to the ligand atoms or groups of polymer chelating agents, they can be divided into the categories shown in Table 3 and 6.
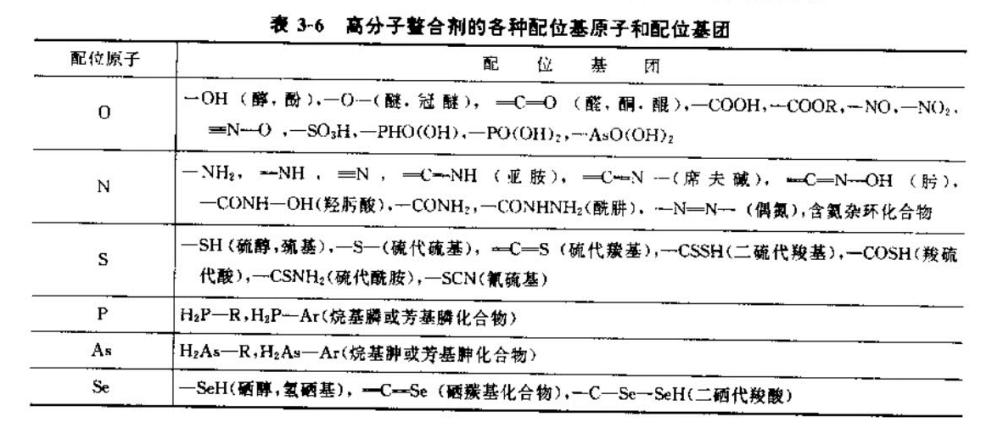
As can be seen from Table 3-6, the coordination atoms are mainly O, P, N, S, As, Se, etc., and the more common ones are organic O, N, S and other coordinating atoms in the group, and the functional group is nothing more than alcohol, ether, aldehyde, ketone, acid, ester group or amine, imine, hydroxylamine, etc. composed of O, N, S Oxygen-containing acids, etc. In many cases, instead of using one coordinating atom or group, two or more coordinating atoms and ligands may be used. This is one of the reasons why there are so many types of polymer chelating agents. As long as the number and distribution of functional groups in the polymer structure are controlled, and the relationship between the structure and function of high molecules is continuously studied, polymer chelating agents for various purposes can be obtained.



 微信扫一扫打赏
微信扫一扫打赏
