Background and overview[1]
2-Amino-4-[(4-fluorobenzyl)amino]-1-nitrobenzene can be used as a pharmaceutical synthesis intermediate. For example, retigabine is prepared, which is an anti-epileptic drug with multiple mechanisms such as a neuronal potassium channel opener and a GABA enhancer. The drug was developed by GlaxoSmithKline/Valeant Pharmaceuticals. It was approved for marketing in the EU on March 29, 2011 (trade name Trobalt), and was approved by the US FDA (trade name Potiga) in the same year for adults. Adjuvant treatment of partial seizures in epilepsy. In addition, phase II clinical trials of this product for the treatment of post-herpetic neuralgia are also ongoing.
Preparation[1]
The preparation of 2-amino-4-[(4-fluorobenzyl)amino]-1-nitrobenzene is as follows:
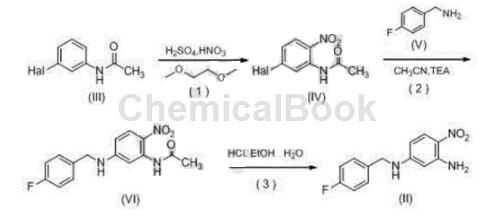
Step 1: Nitration reaction: Combine N-(3-fluorophenyl)acetamide (Compound III, Hal=F) (76.5g, 0.5mol), ethylene glycol dimethyl ether (200mL), concentrated nitric acid ( 65 mL, 1.1 mol) was added to the 1L reaction flask in sequence, stirred, and concentrated sulfuric acid (200 mL, 3.6 mol) was added dropwise at room temperature. After 1.5 hours, the reaction solution was added dropwise to crushed ice, and a white solid precipitated. Then add 1L of water, stir for 15 minutes and then filter. The filter cake is washed with water to obtain 88g of white solid. The obtained crude product is recrystallized with petroleum ether: ethyl acetate = 1.1:1, suction filtered, and the mother liquor is concentrated to obtain white solid N-(5 -Fluoro-2-nitrophenyl)acetamide (compound IV, Hal=F) 74.2g, yield 75%.
Step 2: Condensation reaction: Combine compound (IV, Hal=F) (50g, 0.25mol), acetonitrile (500mL), p-fluorobenzylamine (38g, 0.3mol), and triethylamine (38g, 0.38mol) Add it to a 1L reaction bottle, heat to reflux for 4 hours, stop the reaction, concentrate the reaction solution to dryness, and recrystallize acetonitrile-triethylamine to obtain yellow solid N-{5-[(4-fluorobenzyl)amine]-2-nitrile 72.6 g of phenyl acetamide (compound VI), yield 95%.
Step 3: Hydrolysis reaction: Dissolve compound (VI) (60.6g, 0.1mol) in ethanol (400mL), water (120mL), and concentrated hydrochloric acid (160mL) into a 1L reaction bottle, at 70~80°C Stir for 1 hour. After the reaction solution becomes red and clear, cool to 10°C, adjust the pH to neutral with 4molL-1 sodium hydroxide solution, filter with suction, wash the filter cake with water, and dry with suction to obtain a yellow solid 2-amino-4. -[(4-fluorobenzyl)amino]-1-nitrobenzene 51.2g, yield 98%, mp 112~113°C.
Main reference materials
[1] CN201310543745.8 An intermediate of N1-(4-fluorobenzyl)-4-nitrophenyl-1,3-diamine and its preparation method



 微信扫一扫打赏
微信扫一扫打赏
