Background and overview[1]
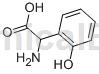
2-Hydroxyphenylglycine can be used as a pharmaceutical synthesis intermediate. If 2-hydroxyphenylglycine is inhaled, move the patient to fresh air; if the skin comes in contact, take off contaminated clothing, wash the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eye contacts, seek medical attention. Separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
The preparation of 2-hydroxyphenylglycine is as follows:
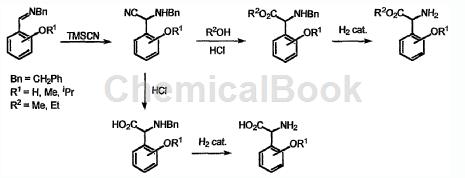 (R1=H)
(R1=H)
a) Dissolve salicylaldehyde and benzylamine (molar ratio 1:1) in ethanol, and reflux the mixture for 2 hours. The reaction was stirred at room temperature for an additional 2 hours and the solvent was removed under reduced pressure to afford the imine in quantitative yield.
b) Add the imine obtained above into anhydrous tetrahydrofuran (THF), slowly add trimethylsilyl cyanide (TMSCN) dropwise, and the molar ratio of biamidization: TMSCN is 1:3. Stir the reaction mixture at room temperature for 40 hours, add saturated ammonium chloride (NH4Cl) solution, extract with diethyl ether, wash the ether phase with water, and finally dry over magnesium sulfate, remove the solvent under vacuum, and quantify it as an oil. α-aminonitrile is obtained.
c) Heat the obtained α-aminonitrile in concentrated hydrochloric acid at 60-70°C for 3 hours. Thereafter, the crude oil was cooled and dried under reduced pressure. After drying, the residue was suspended in acetone, filtered and dried to obtain N-benzyl-2-hydroxyphenylglycine hydrochloride as a powdery solid with a yield of 97%.
d) To a methanol solution of (15% by weight of the mass of the substrate) -benzyl-2-hydroxyphenylglycine N, add a solution of Pd(c) in hydrochloric acid, and then proceed to hydrogen pressure ( 50psi) for 16 hours. The crude product was filtered, and the solvent was removed under reduced pressure to obtain 2-hydroxyphenylglycine with a yield of 96%.
Main reference materials
[1](WO2002102762)NOVELMETHODOFPREPARINGHYDROXYARYLGLYCINES,ALCOXYARYLGLYCINESANDTHEGLYCINATESTHEREOF



 微信扫一扫打赏
微信扫一扫打赏
