Overview[1]
Azide methyl phenyl sulfide is an ether compound that can be used as a pharmaceutical synthesis intermediate. If azidomethylphenyl sulfide is inhaled, move the patient to fresh air; if skin contact occurs, take off contaminated clothing and wash the skin thoroughly with soap and water. If you feel uncomfortable, seek medical attention; if the eyes are clear, In case of contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
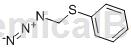
Structure
Preparation[1]
Azide methylphenyl sulfide is prepared as follows:
After bromomethylphenyl sulfide (10b, 1.58 g, 10.0 mmol) was dissolved in dimethylformamide (40 ml), sodium azide (NaN3, 1.3 g, 20.0 Millimoles). Subsequently, the mixture was stirred for 12 hours. Once the reaction is stopped by adding water, the organic compounds are extracted with ethyl acetate. The extracted ethyl acetate solution was treated with anhydrous sodium sulfate, and then subjected to column chromatography (1% ethyl acetate/n-hexane) to obtain the desired compound, azidomethylphenyl sulfide (3c, 1.62 grams, 98%). 1HNMR (500MHz, CDCl3) δ4.54 (s, 2H), 7.37-7.25 (m, 3H), 7.45 (d, J=8.0Hz, 2H).
Apply[1]
Azide methylphenyl sulfide can be used as a pharmaceutical synthesis intermediate, such as the preparation of 4-tert-butyl-1-[(phenylthio)methyl]-1,2,3-triazole: azide Methyl phenyl sulfide (330 mg, 2.0 mmol) and 3,3-dimethyl-1-butyne (4a, 246 mg, 3.0 mmol) were dissolved in acetonitrile (8 ml), and then added Copper iodide (77 mg, 0.4 mmol) and triethylamine (0.056 mL, 0.4 mmol). The mixture was stirred at room temperature for 2 hours and then concentrated under reduced pressure. Subsequently, column chromatography (1% methanol/dichloromethane) was performed to obtain the desired compound, namely, 4-tert-butyl-1-[(phenylthio)methyl]-1,2,3-triazole ( 411 mg, 83%). 1HNMR (500MHz,CDCl3) δ1.23 (s, 9H), 5.90 (s, 2H), 7.45-7.34 (m, 5H), 7.76 (s, 1H).
Main reference materials
[1] CN201280023659.8 F-labeled precursor of PET radioactive medical supplies and its preparation method



 微信扫一扫打赏
微信扫一扫打赏
