Overview[1]
1-(4-bromobenzene)cyclopropylamine can be used as a pharmaceutical synthesis intermediate. If 1-(4-bromobenzene)cyclopropylamine is inhaled, please move the patient to fresh air; if there is skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical treatment if you feel unwell; if In case of eye contact, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Preparation[1]
The preparation of 1-(4-bromobenzene)cyclopropylamine is as follows:
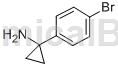
The specific steps are as follows: Dissolve 4-bromobenzonitrile (3.00g, 16.48mmol) and titanium tetraisopropoxide (5.4mL, 18.13mmol) in anhydrous ether (40mL), cool to -78°C, A solution of ethyl magnesium bromide in diethyl ether (3M, 12 mL, 39.88 mmol) was added dropwise. After the dropwise addition is completed, the stirring reaction is continued at this temperature for 10 minutes, and then raised to room temperature for 1 hour. Add boron trifluoride solution in diethyl ether (4.2 mL, 36.26 mmol). After stirring for 1 hour, add 1N dilute hydrochloric acid and diethyl ether. The aqueous phase was collected, basified with 10% aqueous sodium hydroxide solution, and extracted with ethyl acetate. After the organic phase was dried, filtered and concentrated, 1-(4-bromobenzene)cyclopropylamine (1.7g) was obtained with a yield of 48%.
Apply[1]
1-(4-bromobenzene)cyclopropylamine can be used as a pharmaceutical synthesis intermediate. If the following reaction occurs:
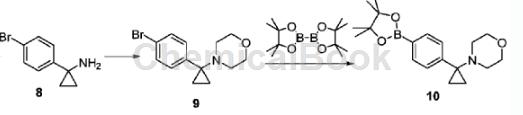
Dissolve 1-(4-bromobenzene)cyclopropylamine (1.70g, 8.015mmol) in N,N-dimethylformamide (20mL), place it in a sealed tube, and add 2,2′-dibromo Diethyl ether (1.4 mL, 8.816 mmol) and N,N-diisopropylethylamine (2.7 mL, 16.03 mmol). The reaction was heated to 100°C and stirred for 16 hours. Cool to room temperature, add ethyl acetate, and wash with water. The organic phase is dried, filtered, and concentrated under reduced pressure. The crude product is separated and purified by column chromatography to obtain intermediate 9 (2.23g), with a yield of 42%. Intermediate 9 (2.23g, 7.902mmol), bis-pinacohol boronate (2.21g, 8.692mmol), [1,1′-bis(diphenylphosphorus)ferrocene]palladium dichloride ( 347 mg, 0.4741 mmol) and potassium acetate (1.16 g, 11.85 mmol) were dissolved in 1,4-dioxane (30 mL), heated to 90°C, and reacted for 16 hours. Concentrate to dryness under reduced pressure, add ethyl acetate, wash with brine, dry, filter, concentrate and separate by column chromatography (petroleum ether/ethyl acetate = 6:1, v/v) to obtain compound 10 (1.75g). The rate is 67%.
Main reference materials
[1] CN106349180 4,5-diphenyl isoxazole derivative as well as preparation method and application thereof



 微信扫一扫打赏
微信扫一扫打赏
