Background and overview[1][2]
C9 aromatics, also known as heavy aromatics, are a variety of aromatic hydrocarbon compositions containing 9 carbon atoms. Their molecular formula is C9H12. C9 aromatics are by-products produced during crude oil processing, mainly from the reforming unit and cracking of refineries. device. C9 aromatic hydrocarbons contain large amounts of mesitylene, mesitylene, mesitylene, o-ethylbenzene, m-ethylbenzene, p-methylethylbenzene, n-propylbenzene and cumene, among which mesitylene, mesitylene and mesitylene The total content is about 80%, which is a valuable resource for the development of fine chemicals and has high added value. The development prospects are projects that are encouraged by the national industrial policy.
In the current production of mesitylene, major manufacturers mainly produce it through distillation, and achieve separation through differences in boiling points. Its shortcomings are: since C9 aromatics also contain a large number of other components, although from the perspective of the industrial chain, the by-products after distillation can still be reused, as a manufacturer of mesitylene, they may not all have them. Complete production equipment for downstream products corresponding to by-products. Therefore, some of the remaining products after distillation can only be sold to other manufacturers at low prices. This results in higher production costs for manufacturers. How to utilize C9 aromatics more rationally so that they can produce more mesitylene is an urgent problem for mesitylene manufacturers to solve.
Physical and chemical properties[1]
Trimethylbenzene is a colorless liquid, insoluble in water, soluble in ethanol, ether and benzene, with a boiling point of 169.35°C. Trimethylbenzene is widely used in fine chemicals. It is an ideal solvent for high-grade paints and the production of hydrogen peroxide by the anthraquinone method. It is also an ideal solvent for the production of trimellitic anhydride, 3,4-dimethylbenzaldehyde, mesitylene and tetramethylbenzene (used in the production of Pyromellitic dianhydride and polyimide resin, etc.) are important raw materials for products.
Trimethylbenzene is an important component of C9 aromatics, mainly derived from platinum reformation of heavy aromatics, xylene isomerization by-products, catalytic cracking of heavy aromatics and ethylene cracking of heavy aromatics (see Table 1). Among them, reformed heavy aromatics are the main source of mesitylene, and high-purity mesitylene can be separated. In recent years, the demand for trimellitic anhydride, a downstream product of trimellitic acid, has increased significantly, which has led to a corresponding increase in the demand for trimellitic acid.
Apply[3]
Trimethylbenzene (1, 2, 4-trimethylbenzene) is an important organic chemical raw material, mainly derived from catalytic reforming of heavy aromatic hydrocarbons. Trimethylbenzene, as a basic organic chemical raw material, is widely used and has many downstream products with high added value. The main uses of trimethylene are as follows: synthesis of trimellitic acid and trimellitic anhydride; production of mesitylene by isomerization; production of mesitylene by disproportionation and isomerization; synthetic fiber 2, 3, 5-trimethylhydroquinone. Among them, the synthesis of trimellitic anhydride from trimellitic acid has become the main use of trimellitic anhydride. The demand for trimellitic anhydride at home and abroad is growing at a rate of 20%. Since the synthesis of trimellitic anhydride requires high purity of trimellitic acid (generally the purity is required to be above 98.5%), now the country basically imports trimellitic anhydride. Trimethylbenzene produces trimellitic anhydride), so the production of trimellitic anhydride with high purity is of great significance for the further production of trimellitic anhydride.
Trimellitic anhydride made from trimethylene:
Using NH4VO3, RbNO3, K2CO3, NH4H2PO4 and TiCl4 as raw materials, using an α-Al2O3 ring with an inner diameter of 4mm, an outer diameter of 6mm and a length of 6mm as a carrier, the active component weight ratio is V2O5: TiO2: P2O5: Rb2O: Catalyst with K2O=10.56:86.71:2.50:0.12:0.11. Take 50ml and put it into a glass reaction tube with an inner diameter of 25mm. Under the operating conditions of reaction temperature 395°C, water vapor:trimethylbenzene=25, catalyst load 34.5g//L·h, and space velocity 3500L/L·h, the relative trimethylbenzene The weight yield of crude trimellitic anhydride in toluene is 107%, and the purity of crude trimellitic anhydride obtained from the trap is 96.8%.
Preparation[2]
A method for producing trimene by isomerizing C9 aromatic hydrocarbons, the steps are as follows:
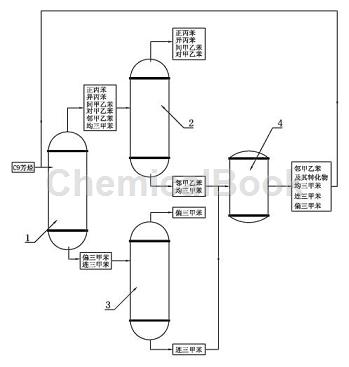
1) The C9 aromatics produced by petroleum cracking with a purity of greater than or equal to 90% are processed in the light removal tower 1, and the working temperature of the light removal tower 1 is controlled to be 166°C~168°C (due to the difficulty of temperature control) Control at a specific precise value, therefore, it is appropriate as long as it fluctuates within a certain range, the same below), so that the boiling point is lower than 166.After the o-methylethylbenzene, m-methylethylbenzene, p-methylethylbenzene, mesitylene, n-propylbenzene and cumene are vaporized and leave the light removal tower 1 from the top of the tower, they enter the first rectification tower 2; the light removal tower 1 The trimene and trithionine retained at the bottom of the tower with a boiling point higher than 168°C are transported to the second distillation tower 3;
2) In the second distillation tower 3, the distillation temperature is 169.5°C~175°C. Trimethylbenzene leaves from the top of the second distillation tower 3 and is collected, and the trimethylbenzene is retained at the bottom of the second distillation tower 3. Toluene;
3) In the first distillation tower 2, the distillation temperature is 162°C~164°C, and mesitylene and o-methylethylbenzene remain at the bottom of the tower;
4) Send the bottom products of the above steps 2) and 3) into the closed isomerization reactor 4 at the same time to perform a catalytic reaction. The catalyst is arranged in the isomerization reactor 4 in the form of a fixed bed. , the reactant stream passes through the fixed bed in liquid form; the pressure in the isomerization reactor is 0.2MPa, the reaction temperature is 260-270°C, and the bottom products of steps 2) and 3) continuously flow through the fixed bed, under the action of the catalyst An isomerization reaction occurs, causing part of the trimethylbenzene and mesitylene to structurally isomerize into mesitylene. At the same time, some o-methylethylbenzene is isomerized into p-methylethylbenzene and m-methylethylbenzene;
5) Continuously input the reaction product obtained in step 4) into the light removal tower 1, and then send it to the second rectification tower 3 after light removal, where it is rectified in the second rectification tower 3 to obtain trimene. The bottom product is sent again to the isomerization reactor 4 for recycling reaction.
Methylation of BTX aromatic hydrocarbons to synthesize trimethylene
Preparation of molecular sieve catalyst:
NaZSM-5 with SiO2/Al2O3=55, NaZSM-22 with SiO2/Al2O3=30, NaMCM-22 with SiO2/Al2O3=50, NaMCM-49 with SiO2/Al2O3=25, were prepared by hydrothermal synthesis. NaMCM-56 molecular sieve with SiO2/Al2O3=25 and Naβ molecular sieve with SiO2/Al2O3=30, the obtained molecular sieve is exchanged with ammonium chloride, washed and roasted, then pressed into tablets and sieved to obtain particles of a certain mesh size to obtain hydrogen type molecular sieve. Catalysts HZSM-5, HZSM-22, HMCM-22, HMCM-49, HMCM-49, HMCM-56 and Hβ are used as catalysts for alkylation to synthesize trimethylene.
BTX aromatic hydrocarbons and methylating agent are used as raw materials, and trimylene is synthesized through methylation reaction under the action of acid catalyst. Wherein, the BTX aromatic hydrocarbon is any one or more of benzene, toluene, o-xylene, m-xylene, p-xylene and mixed xylene, preferably benzene, toluene and xylene; the methylating agent It is any one or more of methanol, dimethyl ether and methyl chloride, preferably methanol; the methylation reaction method adopts fixed bed reaction or kettle type reaction, preferably fixed bed continuous reaction or kettle type continuous reaction; the reaction product All the C6~C9 aromatic hydrocarbon materials other than mesitylene are recycled back to the reactor, and are further methylated or isomerized on the same catalyst to regenerate mesitylene.
Main reference materials
[1] Zhang Weijiang, Zhang Xuemei, Han Zhenwei, Sun Xinghua, Wang Fengdong, & Zhang Junbao et al. (2002). Process of producing mesitylene from mesitylene. Journal of Chemical Engineering, 53(3), 274-279.
[2] Guo Li, An Hong, Guo Qiangguo, & Xu Weili. (2007). Application of high-purity mesitylene production technology. Journal of Lanzhou Petrochemical Vocational and Technical College, 7(2), 1-4.
[3] Li Suidang, & Fu Jiquan. (2008). Catalytic oxidation of trimethylene to synthesize trimethylbenzoquinone. Petrochemical Technology and Application, 26(3), 234-236.



 微信扫一扫打赏
微信扫一扫打赏
