Background and overview[1]
Meta-tolyl magnesium bromide can be used as an intermediate for pharmaceutical and chemical synthesis. If m-tolyl magnesium bromide is inhaled, move the patient to fresh air; if skin contact occurs, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel unwell; if contact with eyes, remove The eyelids should be separated, rinsed with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Structure
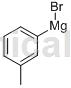
Apply[1]
Meta-tolyl magnesium bromide can be used as an intermediate for pharmaceutical and chemical synthesis. If the following reaction occurs:
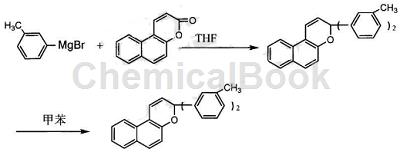
The specific steps are:
Step 1: Synthesis of naphthopyran-2-one
In a 100ml three-necked flask, add 0.1mol 2-hydroxy-1-naphtaldehyde, 0.2mol anhydrous acetic anhydride, and 0.2mol anhydrous sodium acetate, raise the temperature to 160°C, reflux for 6 hours, and monitor with TLC. Wait until the reactants After the point disappears, stop heating. After cooling, add sodium carbonate aqueous solution with a mass percentage concentration of 10% to neutralize the unreacted acetic anhydride. Adjust the pH to 7, add chloroform, dissolve the generated solid, separate the liquids, and use anhydrous magnesium sulfate. Dry, filter, evaporate the chloroform, add petroleum ether to produce a precipitate, filter, and dry the solid. The m.p. of the naphthopyran-2-one obtained is 115 to 116°C, and the yield is 88%.
Step 2: Synthesis of 2,2-bis(3-methylphenyl)naphthopyran photochromic compound
Add 0.01 mol of naphthopyran-2-one prepared in the first step above dissolved in 16 ml of anhydrous tetrahydrofuran dropwise to m-tolyl magnesium bromide. After the dropwise addition, continue to reflux for 6 hours, and monitor with TLC. , until the raw material point disappears, stop the reaction, evaporate the tetrahydrofuran, add an aqueous ammonium chloride solution with a mass percentage concentration of 22%, hydrolyze the unreacted Green’s reagent, add toluene for extraction, dry with anhydrous magnesium sulfate, and filter the toluene formed The solution was refluxed in the dark for 4 hours. The toluene was evaporated and separated by column chromatography to obtain 2,2-bis(3-methylphenyl)naphthopyran photochromic compound with a yield of 50%.
Preparation[1]
The preparation of m-tolyl magnesium bromide is as follows: under nitrogen protection, add 0.03 mol magnesium shavings to a 100 ml three-necked flask, add anhydrous ether so that the magnesium shavings are just covered, and add 0.2 ml of 0.04 mol3 dropwise while stirring. – A solution of methyl bromobenzene and 15 ml of anhydrous ether. Warm the reaction bottle until the reaction begins, and then add dropwise the remaining solution of 0.04 mol of 3-methyl bromobenzene and 15 ml of anhydrous ether. Keep Reflux, after the dropwise addition is completed, continue to reflux until the reaction of the magnesium chips is completed, to prepare m-tolyl magnesium bromide.
Main reference materials
[1] CN201110275394.8 Preparation method of aryl-substituted naphthopyran photochromic compounds



 微信扫一扫打赏
微信扫一扫打赏
