Background and overview[1][2]
Phenoxyacetic acid is a very important chemical. It and its downstream products are widely used pesticides and pharmaceutical products. It has a long history of use. Years of use have proven that it has low toxicity, high efficiency and safety. Good features are widely accepted by people. However, the production process of these chemicals is still relatively backward, and most of them are still in the state of their originally developed production processes, which have many shortcomings such as low yields, high waste emissions, and large amounts of waste water.
The traditional preparation method is to pass chlorine gas through phenoxyacetic acid under the catalysis of Lewis acid. The reaction temperature required by this method is 45℃~75℃. The reaction process must strictly control the chlorine gas feeding speed and has high requirements on equipment. The conditions are harsh, resulting in high production costs, and the method has low reaction selectivity and serious environmental pollution. In order to avoid environmental pollution problems, the existing technology proposes to use glacial acetic acid and sodium hypochlorite as chlorination reagents to prepare p-chlorophenoxyacetic acid. This method requires p-Chlorophenoxyacetic acid can be prepared through multi-step reactions. The preparation cost is high and the yield is only 35% to 41%.
Existing technology Glacial acetic acid and sulfonyl chloride are used as chlorination reagents to prepare p-chlorophenoxyacetic acid. The reaction temperature is 75°C. Phenoxyacetic acid can be chlorinated into 2,4-dichlorophenoxyacetic acid with a yield of 74%. . This process uses expensive sulfonyl chloride as raw material, which results in higher production costs and stricter conditions. The entire device must be airtight, otherwise it will affect the chlorine gas utilization rate. Since Lewis acid is easy to hydrolyze, the reaction is required to be carried out under anhydrous conditions; The temperature is appropriate; if it is too high, by-products may be produced; if it is too low, the reaction time will be prolonged; post-processing will be troublesome.
Physical and chemical properties and structure[1]
Phenoxyacetic acid is a white flake or needle-shaped crystal that naturally exists in cocoa beans. It has a sour sweet aroma and a honey-like taste. Appearance: crystalline solid, melting point 98~100℃, boiling point 285℃ (decomposition). According to relevant reports, the acute toxicity data of phenoxyacetic acid: oral LD50>5g/kg (rat), skin test LD50>5g/kg (rabbit). It can be obtained by condensing phenol and monochloroacetic acid in a sodium hydroxide solution to generate sodium phenoxyacetate, and then acidifying it.
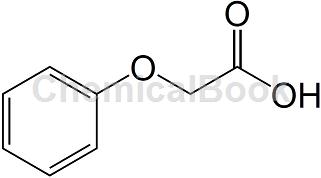
Phenoxyacetic acid
Apply[3]
Phenoxyacetic acid derivatives such as 2,4-D acid, 2-methyl-4-chloride and other herbicides have a very broad market demand and have been around for decades. The traditional production process is to use phenol chlorination Afterwards, it is synthesized with sodium chloroacetate, and chlorinated phenol-containing wastewater is produced during the production process, which has a serious impact on the production environment and the treatment of three wastes. In the process of first chlorinating phenol and then condensing it to synthesize chlorophenoxyacetic acid herbicides, since the chlorine-containing phenols produced by chlorination of phenols are difficult to separate, the chlorine-containing by-product phenols have a greater impact on the quality of the product. In addition, in the reaction of synthesizing phenoxyacetic acid, the chlorinated creosol contained in the wastewater produced has a great impact on the post-treatment of the wastewater, which is not conducive to biochemical degradation and discharge.
Allyl phenoxyacetate, also known as pineapple ether, is a monomeric spice with a pineapple-like fruit aroma accompanied by honey notes. Can be used in daily fragrance and food flavor formulations. Pineapple ether is generally obtained through an esterification reaction of phenoxyacetic acid and allyl alcohol in the presence of an acidic catalyst and a water-carrying agent.

Preparation[2]
The synthesis method of phenoxyacetic acid mainly adopts Williamson synthesis method. In recent years, various new synthesis methods such as phase transfer catalytic method have also been developed. Wu Xiaowei et al. once reported the use of wet synthesis and microwave method to synthesize phenoxyacetic acid, with a yield of 84.2% and a measured melting point of 97-98°C. Later, Wang Cunde et al. expanded and quickly synthesized a series of aryloxyacetic acids using microwaves. The rate can reach 86.8%-94.1%. Li Jitai et al. reported a two-phase synthesis method in which phenoxyacetic acid was prepared from the condensation of phenol and chloroacetic acid in a benzene-sodium hydroxide aqueous solution. The product yield was 96.4%.
Compared with the classic method, this method has a lower reaction temperature, simpler operation, high product yield, and good reproducibility. Huang Xiaoling et al. [Pesticides, 1989, 28(6):21] also reported the use of 2-fluoro-4-methylbenzyltrimethylammonium bromide (fluoroquaternary ammonium) as a catalyst to catalyze the condensation of arylphenols and chloroacetic acid. reaction, the yield was 75%. The melting point of the product is consistent with literature values. The advantages of using fluoroquaternary ammonium catalytic method to synthesize phenoxyacetic acid over liquid-liquid, liquid-solid and three-phase catalytic methods are: no organic solvents, short reaction time, high yield and simple operation.
Li Yingjun et al. [Journal of Northwest Normal University (Natural Science Edition), 1986, 2:47-49] have reported the synthesis of aryloxyacetic acid using a solid-liquid phase transfer catalytic method. The reaction was catalyzed by the solid-liquid phase transfer catalyst polyethylene glycol (PEG-400). Qing Fengling et al. [Chemical Reagents, 1989, 11(4):250] reported the synthesis of aryloxyacetic acid under three-phase catalytic conditions using a homemade polymer catalyst-polystyrene-supported polyethylene glycol. Huang Shiwei [Chemical Reagents, 1992, 14(5):313] and others also reported a method for rapid synthesis of aryloxyacetic acid, using a very small amount of water, with a yield as high as 98%.
Liang Ying et al. screened out the optimal process conditions through orthogonal experiments as phenol: chloroacetic acid: sodium hydroxide = 0.9:1.1:2.4, reacted at 102°C for 5 hours, and the yield was 69%.
Main reference materials
[1]Li Jinchang, & Wang Lu. (2001). Determination of phenoxyacetic acid and 2,4-dihydrophenoxyacetic acid by solid-phase extraction and enrichment high-performance liquid chromatography. Analytical Chemistry, 29(5), 580 -582.
[2]Liang Ying, & Shi Xiaopeng. (2001). Research on the synthesis of phenoxyacetic acid. Applied Chemical Engineering, 30(4), 31-32.
[3]Wu Jie, Ni Peizhou, Jiang Zhenzhou, & Li Min. (2002). Synthesis of phenoxyacetic acid compounds and their lipid-lowering activity. Journal of China Pharmaceutical University, 33(6), 478-482.



 微信扫一扫打赏
微信扫一扫打赏
