Background and overview[1][2]
The Caspase family is a large class of apoptosis regulatory factors and is the initiator and final executor of apoptosis. Apoptosis initiating factors are composed of caspase-2, caspase-8, caspase-9 and caspase-10, and apoptosis execution factors are composed of caspase-3, caspase-6 and caspase-7. Studies have shown that in the caspase family, caspase-3 is the most critical apoptosis-executing protease downstream of the “waterfall” of the caspase cascade. It exists in the form of zymogen in normal cells and is affected by apoptosis-stimulating factors (ischemia, intracellular Activated after calcium overload, etc.), activated caspase-3 can activate nuclear factors, cytoskeletal proteins and DNA cleavage enzymes, etc., causing changes in cell morphology such as cell shrinkage, DNA cleavage, chromatin condensation and apoptotic bodies. formation, etc., ultimately leading to cell apoptosis. Caspase-3 plays a final hub role in the apoptotic program initiated by various factors. During cerebral ischemia, the death receptor-mediated apoptosis pathway activates caspase-8, which further activates its downstream effector protease caspase-3 and living mitochondrial apoptosis pathway to cause cell apoptosis. Studies have shown that inhibiting caspase enzyme activity can prevent the development of neuroapoptosis, reduce the degree of cerebral ischemic damage, and reduce the scope of infarction.
Emricasan (1) is a potent, oral caspase inhibitor with the chemical name N-[2-(tert-butyl)phenyl]-2-oxoglycine Acyl-N-[(1S)-1-(carboxymethyl)-2-oxo-3-(2,3,5,6-tetrafluorophenoxy)propyl]-L-alaninamide. Enricin is a first-in-class new drug designed to reduce the activity of enzymes that mediate inflammation and cell death or apoptosis. By reducing the activity of these enzymes, Enrican may block the progression of the entire liver disease. Conatus Pharmaceuticals announced headline-grabbing results from a Phase II clinical trial of emricasan for the treatment of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. The drug is an orally available pan-caspase inhibitor. 38 patients with non-alcoholic fatty liver disease and non-alcoholic steatohepatitis took the drug 25 mg twice a day. After 28 days, compared with the placebo group, when the trial reached the primary endpoint, alanine aminotransferase in the treatment group was reduced by 39% and in the placebo group was reduced by 14%. Three biomarkers in serum, caspase-cleaved cytokeratin 18 (cCK18), full-length cytokeratin 18, and caspase 3/7, were also significantly reduced compared with baseline. cCK18 decreased by 30%, indicating that this drug can effectively reduce inflammation. Enricana is safe and well tolerated, with no serious drug-related adverse reactions.
Pharmacological effects and applications[3]
Enlicasan is a potent and orally administered caspase inhibitor, an investigational drug invented by Conatus Pharmaceuticals in the United States. It is used to treat cirrhosis or various liver injuries. For patients with severe liver injury, Phase II clinical treatment trials have been completed with excellent efficacy. Additionally, there were no adverse effects on blood lipid levels or insulin sensitivity in patients treated with enricaxen, who were at typical risk for cardiovascular disease. As Enricana is further used in experimental studies on the therapeutic effect of various liver diseases, it is expected to provide a good medical foundation for the treatment of progressive stages of liver diseases.
Preparation [3-4]
Method 1: Condensation of N-benzyloxycarbonyl-L-alanine succinimide ester and L-aspartic acid-β-tert-butyl ester to obtain dipeptide, which is activated by isobutyl chloroformate. React with diazomethane, replace bromine with hydrogen bromide in acetic acid solution, replace bromine with tetrafluorophenol, then reduce with sodium borohydride, remove benzyloxycarbonyl protection from Pd-C in hydrogen environment to obtain intermediate 7; o-tert-butylaniline and chlorine Methyl oxalate is amidated, and then the methyl ester is hydrolyzed to obtain intermediate 10, which is then condensed with 7 to form an amide, and then undergoes oxidation to remove the tert-butyl ester protection to obtain the target compound Enrican 1.
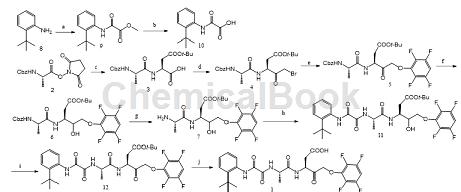
The specific steps are as follows:
Step 1: (a) Isobutyl chloroformate (1.1eq), triethylamine TEA (1.1eq), add at 0°C, chamber
Temperature 1 h; (b) 1N lithium hydroxide solution (1.2eq), 1,4-dioxane solvent, room temperature 1 h; (c) Bis(trimethylsilyl)trifluoroacetamide BSTFA (2.0eq), L-aspartic acid-β-tert-butyl ester (1.0eq), acetonitrile, room temperature 24h; (d) i. Isobutyl chloroformate (1.5eq), N-methylmorpholine (1.6 eq), THF, -10 ℃, 20 min; ii. Diazomethane ether solution (2.5eq), 0 ℃, 15 min; iii. 48% hydrogen bromide acetic acid solution, THF, 0 ℃ – room temperature, 30 min; ( e) 2,3,5,6-tetrafluorophenol (1.2eq), KF (4.0eq), THF, room temperature 16 h; (f) NaBH4 (1.1eq), THF, 0 ℃, 1 h; (g) 10% Pd-C/H2, methanol, room temperature 2 h; (h) 10 (1.0eq), HoBt (1.05eq), EDCI (1.4eq), N-methylmorpholine (1.05eq), dichloromethane, Room temperature 16 h; (i) Iodobenzene diacetate (3.8eq), 2,2,6,6-tetramethylpiperidine-oxynitride TEMPO (0.2eq), dichloromethane, room temperature 16 h; (j) Three
Fluoroacetic acid, methylene chloride, anisole, room temperature for 1 h.
Step 2: Preparation and purification of 4-(3-piperidyl)aniline tartrate: Dissolve the free amine product from the previous step in 300mL of absolute ethanol, and add 1 mol/L (L) within 5 minutes – Tartaric acid ethanol solution (28 mL, 0.028 mol), after addition, the reaction solution was refluxed for 2 hours, then slowly cooled down, frozen for crystallization, filtered after the crystallization was complete, and the filter cake was washed with absolute ethanol (100 mL × 2) and dried to obtain 5.9g of light yellow solid, yield 60%.
Method 2: Using pyruvic acid as raw material, reacting with L-alanine methyl ester through acyl chloride reactionCombine, then hydrolyze the methyl ester, continue the acid chlorination reaction, and react with (S)-3-amino-5-(2,3,5,6-tetrafluorophenoxy)-4-oxopentanoic acid tert-butyl ester After condensation, the pyruvate ketone is oxidized to obtain a carboxylic acid derivative. The acid chloride reaction is continued, and it is condensed with o-tert-butylaniline to form an amide. The tert-butyl protection is removed with trifluoroacetic acid to obtain the target product 1.
Method 3: A method for synthesizing enricarsen, including the following steps:
A) Preparation of intermediate (I): First, acyruvate is reacted with acyl chloride reagent in the solvent used for acyl chlorination reaction to obtain pyruvyl chloride, and then pyruvyl chloride and L-alanine methyl ester are mixed in The condensation reaction is carried out in a system of the solvent used for the condensation reaction and the acid-binding agent base to obtain intermediate (I);
B) Preparation of intermediate (II): First, the intermediate (I) obtained in step A) is put into a system composed of a base, a solvent for the hydrolysis reaction and water to perform a hydrolysis reaction to obtain a carboxylic acid derivative. The carboxylic acid derivative is subjected to an acid chlorination reaction with an acid chlorination reagent in a solvent for the acid chlorination reaction to obtain an acid chloride derivative, and then the acid chloride derivative is reacted with (S)-3-amino-5-(2,3, 5,6-Tetrafluorophenoxy)-4-oxopentanoic acid tert-butyl ester is subjected to a condensation reaction in a system of a solvent for the condensation reaction and an acid-binding agent base to obtain intermediate (II), wherein, the The solvent used in the hydrolysis reaction is methanol, ethanol, isopropanol, n-propanol or tert-butanol;
C) Preparation of finished products: First, the intermediate (II) obtained in step B) is put into a system composed of an oxidizing reagent, a solvent for the oxidation reaction and water to perform an oxidation reaction to obtain a carboxylic acid derivative. The acid derivative is subjected to an acid chlorination reaction with an acid chlorination reagent in a solvent used for the acid chlorination reaction to obtain an acid chloride derivative, and then the acid chloride derivative is mixed with 2-tert-butylaniline in a solvent used for the condensation reaction and an acid binding agent base. A condensation reaction is carried out in the system to obtain a tert-butyl ester derivative. The tert-butyl ester derivative is then treated with trifluoroacetic acid to perform a hydrolysis reaction to obtain the finished product Enricarsen.
Main reference materials
[1] Enricaxen treats non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
[2] CN201710021142.X Application of Enricana in the preparation of drugs for the treatment of ischemia/reperfusion injury
[3] CN201510393792.8 A kind of synthesis method of Enricana
[4] Synthesis of enricarsen, a caspase inhibitor



 微信扫一扫打赏
微信扫一扫打赏
