Background and overview[1]
4-[3-(4-hydroxybutyl)-4,4-dimethyl-2,5-dioxo-1-imidazolidinyl]-2-(trifluoromethyl)benzonitrile Used as pharmaceutical and chemical synthesis intermediates. If 4-[3-(4-hydroxybutyl)-4,4-dimethyl-2,5-dioxo-1-imidazolidinyl]-2-(trifluoromethyl)benzonitrile is inhaled, get Move the patient to fresh air; in case of skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eye contact occurs, separate the eyelids and rinse with running water or normal saline Rinse and seek medical attention immediately; if ingested, rinse mouth immediately. Do not induce vomiting and seek medical attention immediately.
Structure
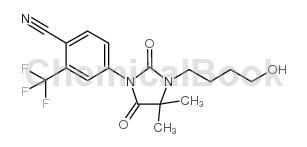
Preparation [1]
Method 1: 4-[3-(4-hydroxybutyl)-4,4-dimethyl-2,5-dioxo-1-imidazolidinyl]-2-(trifluoromethyl) The synthesis of benzonitrile is as follows:
Step 1: Synthesis of 4-amino-2-(trifluoromethyl)benzonitrile. Under a nitrogen atmosphere, add 377 mg copper (II) cyanide 14C (9GBq) and 1.0732g 4-bromo-3-(trifluoromethyl) into 8 ml dimethylformamide, mix, reflux for 4 hours, and cool to 0 ℃ and dilute with 20ml acetone. The insoluble portion was filtered off, and the filtrate was concentrated under reduced pressure at 700°C. The residue was dissolved in dichloromethane, filtered, and the filtrate was concentrated under reduced pressure. Purification of benzonitrile (14C) by silica chromatography (eluent: dichloromethane-cyclohexane (70-30)) afforded 0.558 g (6.62 GBq) of the expected product.
Step 2: Synthesis of 4-isocyanato-2-(trifluoromethyl)benzonitrile. 182.4 mg of benzonitrile (14C) (0.97 mmol), 2 ml of dioxane and 1 ml of toluene of 20% phosgene were mixed together under a nitrogen atmosphere, and the solution was heated at 60 °C for 22 h and then at 60 Decompress concentratedly at ℃. The isocyanate was used as such in the next step.
Step 3: 4-(4,4-dimethyl-3-(4-hydroxybutyl)-5-imino-2-oxo-1-imidazolidinyl)-2-(trifluoromethyl base) benzonitrile synthesis. Add 1.5ml dichloromethane (on siliceous rock NK 30), 4-(4,4-dimethyl-3-(4-hydroxybutyl)-5-imino-2-oxo- A solution of 1-imidazolidinyl)-2-(trifluoromethyl)-(5-3H)-propyl benzonitrile in 1.5 ml dichloromethane and 150 μl triethylamine, the isocyanate of step 2), and the mixture was Stir at 20°C for 1 hour and concentrate under reduced pressure. The imine was used as such in the next step.
Step 4. 4-[3-(4-hydroxybutyl)-4,4-dimethyl-2,5-dioxo-1-imidazolidinyl]-2-(trifluoromethyl) Synthesis of benzonitrile. Add 5 ml methanol and 1.2 ml 1N hydrochloric acid to the imine from step 3, reflux the mixture for 40 minutes, then reach 200°C again and dilute with 10 ml water. Extract with dichloromethane, wash the extract with water and concentrate under reduced pressure. The crude product was purified by silica chromatography (eluent: diethyl ether-acetonitrile-cyclohexane (50-15-35)) to give 289 mg (1.26 GBq) of the expected product.
Main reference materials
[1] WO2009097996.USE OF SUBSTITUTED PHENYLIMIDAZOLIDINES FOR PRODUCING MEDICAMENTS FOR TREATING METABOLIC SYNDROME



 微信扫一扫打赏
微信扫一扫打赏
