Background and overview[1]
At present, anti-infective drugs have become the third largest class of drugs in the world after cardiovascular system drugs and central nervous system drugs, occupying an important share in the world market. Itraconazole, as a representative variety of anti-infective drugs, has become the best-selling variety in the world in 2006. With its unique mode of action and excellent efficacy, its market share is on the rise. cis 2 (2,4 dichlorophenyl) 2 ([1,2,4]triazole 1 methyl) [1,3]dioxolane 4 methyl Mesylate is an important intermediate in the synthesis of antifungal drugs and a necessary intermediate in the synthesis of itraconazole. It is of great significance to the research and development of new synthesis processes.
cis 2 (2,4 dichlorophenyl) 2 ([1,2,4] triazole 1 methyl) [1,3]dioxolane 4 Methyl methanesulfonate is very difficult to synthesize because it involves a stereoscopically active structure. At present, the international synthesis process mainly uses dichloroacetophenone as the raw material, which is brominated, catalyzed by concentrated sulfuric acid, condensed with glycerol and then esterified. The bromoester is obtained and then reacted with 1,2,4 triazine under the catalysis of sodium methoxide. Azole condensation, hydrolysis, column chromatography separation of isomers to obtain cis-alcohol, and then esterification with methanesulfonyl chloride to obtain methanesulfonic acid active ester; or using bromo ester with 1,2,4 triazine under the catalysis of sodium hydride Azole condensation, hydrolysis, column chromatography separation of isomers to obtain cis-alcohol, and then esterification with methanesulfonyl chloride to obtain methanesulfonic acid active ester.
Process route ① has the following shortcomings: first, free radical bromination of 2,4-dichloroacetophenone is performed. Since there are three hydrogen atoms on the methyl group of acetophenone, deep bromination is inevitable, which is not economical and Environmentally friendly; when condensing with glycerol, concentrated sulfuric acid is used as a catalyst for condensation, which inevitably produces a large amount of waste acid, and the post-processing is cumbersome; at the same time, cis alcohol and its isomers are subjected to column chromatography after hydrolysis The method is used for separation and purification, and the high cost is not conducive to large-scale production; the process route ② uses sodium hydride to catalyze the condensation of bromoester and 1,2,4 triazole, and separates and purifies cis-alcohol through column chromatography, because sodium hydride is expensive It can spontaneously ignite in humid air, and can undergo strong chemical reactions when exposed to acids, water, halogens and oxidants, easily causing combustion or explosion. Therefore, industrial production costs are high and dangerous. At the same time, column chromatography is not conducive to industrial production.
Preparation[1]
A cis 2 (2,4 dichlorophenyl) 2 ([1,2,4] triazole 1 methyl) [1,3]dioxolane 4. The synthesis method of methylmethanesulfonate includes the following steps: (1) First, use m-dichlorobenzene as raw material, and carry out Fu Ke reaction with bromoacetyl bromide; (2) Then in the heteropolyacid TiSiW12O40/SiO2 Condensation with glycerol under catalysis; (3) Esterification with benzoyl chloride; (4) Condensation with 1,2,4 triazole under sodium carbonate catalysis, hydrolysis and recrystallization to obtain cis-alcohol; (5) Using formazan Esterification of sulfonyl chloride gives the product. The reaction equation is as follows:
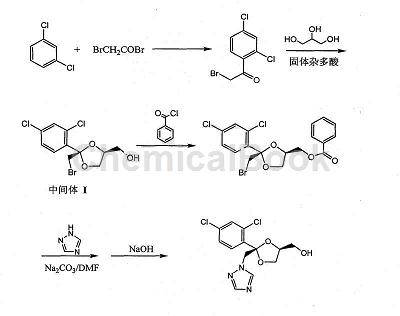
Synthesis of 1)2 bromine 1 (2,4 dichlorophenyl)ethanone
Put 150g (1.12mol) anhydrous aluminum trichloride into a 500mL three-necked flask, add 147g (1.00mol) m-dichlorobenzene, stir at room temperature, and slowly add 201g (1.00mol) bromoacetyl bromide dropwise. After the addition is completed, continue stirring at room temperature for 30 minutes, slowly raise the temperature to 50-55°C, and stir at this temperature for 5 hours. Pour the reaction solution into ice water to thaw, cool to room temperature, and divide into two parts with 500 mL of methylene chloride. Extract twice, combine the dichloromethane extracts, wash with water until neutral, dry with anhydrous sodium sulfate, filter to recover the solvent, and re-condensate the residue to obtain 240g of a white irritating solid with a yield of 90.0%.
2) Synthesis of Intermediate I
Add 268g of the solid obtained in the previous step, 92g of glycerol, 1.3g of toluene and catalyst TiSiW12O40/SiO2 into a 1000mL four-necked flask, and heat to reflux and azeotropic dehydration (subject to the fact that no water can be separated). After the reaction, the catalyst is filtered and recovered, and recycled after regeneration. The mother liquor is washed with potassium hydroxide, dried with anhydrous MgSO4, and the solvent is recovered under reduced pressure to obtain a yellow oil (can be analyzed by GC), with a yield of 94%.
3) Synthesis of cis-bromoester
Add triethylamine to a 1000mL four-necked flask, then pump in 341g of the above bromide, stir and dissolve, then cool to below 10°C, add benzoyl chloride dropwise, and finish dropping in about 3 hours. After the dripping is completed, continue the reaction at 30°C for 2 hours, recover triethylamine under reduced pressure, then add water and chloroform, separate layers, and separate the organic phase.Wash with hydrochloric acid, dry with anhydrous Na2SO4, recover chloroform under reduced pressure to obtain an oily substance, add methanol, stir and precipitate a solid, filter and dry to obtain a crude product, and then recrystallize with methanol to obtain 260g of cis-bromoester, yield 58 %, mp: 117 120°C.
4) Synthesis of cis-alcohol
Add DMF, 446g cis-bromoester, 69g 1,2,4 triazole, and 53g sodium carbonate into a 1000mL four-necked flask, stir and heat to reflux, then add water and 30% alkali solution, and keep warm for 2 hours Afterwards, the temperature was lowered to 30°C, stirred for 1 hour, centrifuged, washed and dried to obtain crude cis-alcohol. The crude product was recrystallized with toluene and methanol, filtered and dried to obtain 205g of cis-alcohol fine product, yield 62%, mp: 135 140°C.
5) Synthesis of methanesulfonic acid active ester
Add triethylamine to a 1000mL four-necked flask, then add cis-alcohol, stir to dissolve and cool to 5°C, then add methylsulfonyl chloride dropwise, raise the temperature to 25°C after addition, keep the temperature for 5 hours and then recover the solvent. Add an appropriate amount of water and stir for 5 hours, then filter, wash, dry, and recrystallize to obtain 359g of cis 2 (2,4 dichlorophenyl) 2 ([1,2,4] triazole 1 Methyl) [1,3]dioxolane 4 methyl methanesulfonate, a white or off-white solid, yield 88%, mp: 96 100°C.
Main reference materials
[1] CN101302213. cis-2-(2,4-dichlorophenyl)-2-([1,2,4]-triazole-1-methyl)-[1,3]bis Preparation method of oxolane-4-methyl methanesulfonate



 微信扫一扫打赏
微信扫一扫打赏
