Background and Overview
4-Iodoanisole, also known as p-iodoanisole, is a halogen substituted anisole, which is a yellow to brown powder at room temperature. 4-Iodoanisole is stable in closed containers and unstable when exposed to light.
Synthesis method[1][2][3]
Synthetic path 1:
Weigh p-nitrobromobenzene (50.5mg, 0.25mmol), KI (124.5mg, 0.75mmol), cuprous oxide (3.6mg, 0.025mmol), 1,6-hexanediamine (5.81mg, 0.05 mmol), and add it to a 25 ml Schlenk bottle in turn, add refined ethanol (1.5 ml), and place it in a 110°C oil bath to react for 30 hours. After the reaction is completed, filter the reaction solution through a silica gel column into a 10 ml volumetric flask. , and quantify the volume of the solution to the specified mark with ethyl acetate. The yield of 4-nitroiodobenzene is about 85%. The general reaction formula is as follows.

Synthesis path 2:
In a 250ml three-necked flask, add 0.05mol iodine and 0.02mol H5IO6 to 100ml n-butyl ether, and add 0.1mol anisole. Raise the temperature to 100°C with stirring, heat for one hour, cool to zero, crystallize, filter and dry to obtain 4-iodoanisole.
Synthetic path 3:
Using anisole as the starting material, add anisole (118.9mg, 1.10mmol), iodine (149.8mg, 0.59mmol), and bismuth nitrate (11.7mg, 0.024mmol), bismuth chloride (11.0mg, 0.035mmol) was stirred in 1ml acetonitrile, the reaction was stopped after 6 hours, 10ml of methylene chloride was added to the reaction mixture, and filtered. Then wash with 0.5M sodium thiosulfate aqueous solution (10ml × 3 times) until excess iodine is washed away. The aqueous phase is extracted once with 10ml of methylene chloride. The organic phases are combined, dried over anhydrous magnesium sulfate, filtered, and reduced pressure. The dichloromethane was removed by distillation, and 233.3 mg of the product 4-iodoanisole was obtained by column chromatography, with a yield of 90%.
Application areas[4][5][6]
1. Compound air disinfection preparation
The air disinfectant is prepared from the following raw materials by weight: 0.5 parts of methylene biscyclohexylamine hexanediamine, 2 parts of glutaraldehyde, 5 parts of polyhydroxyethyl methacrylate, 2 parts of zinc acetate, 5 parts of p-iodoanisole, 1 part of macrophyllin, 3 parts of sodium carbonate, and 10 parts of water.
2. Preparation of acylurea compounds
Using 4-iodoanisole and urea as raw materials, palladium acetate as catalyst, triethylamine as base, iron pentacarbonyl as CO release source, 4-iodoanisole and urea are coupled under mild conditions The carbonylation reaction produces the urea compound 4-methoxybenzoylurea.
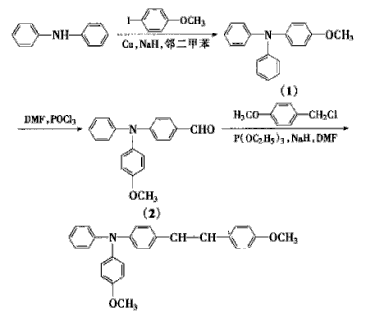
3. Preparation of charge transport materials
Triphenylamine compounds can form amine ion radicals under the action of an electric field, giving them high hole mobility and good hole transport properties. 1-(4-methoxy)phenyl-2- [4′-(4″-methoxy)triphenylamine]-ethylene has unique electrochemical properties, a wide range of applications, and excellent performance when used as charge transport materials. Preparation of 1-(4 from 4-iodoanisole The synthetic route of -methoxy)phenyl-2-[4′-(4″-methoxy)triphenylamine]-ethylene is shown in the figure below. Under nitrogen protection, using diphenylamine, p-iodoanisole and p-methoxybenzyl chloride as raw materials, the charge transport material 1-(4-methoxy )Phenyl-2-[4′-(4″-methoxy)triphenylamine]-ethylene.
Main reference materials
[1] Feng Xiujuan, Bao Ming, Yu Xiaoqiang, a method for preparing iodoaromatic hydrocarbons from iodination of bromine on the aromatic ring, CN 201410334551, application date 2014-07-15
[2] Liu Guobin, Li Yuanqiang, Kuang Tongtao, a monoiodo synthesis method for preparing aromatic hydrocarbons or aromatic heterocyclic compounds, CN 200910045552, application date 2009-01-20
[3] Wan Shun, Lu Wenjun, Wang Rixin, Preparation method of aromatic hydrocarbon iodine compounds, CN 200510111515, application date 2007-12-19
[4] Shao Xuxia, an improved air disinfection preparation, CN 201310478841, application date 2013-10-15
[5] Gao Ziwei, Zheng Shaohua, Zhang Weiqiang, a mild carbonylation and arylation reaction method for urea compounds, CN 201710609757, application date 2017-07-25
[6] Li Haihua, Wen Libin. Synthesis of charge transport material 1-(4-methoxy)phenyl-2-[4′-(4″-methoxy)triphenylamine]-ethylene[ J]. Modern Chemicals, 2006, 26(5): 29-30.



 微信扫一扫打赏
微信扫一扫打赏
