Overview
Phenylhydrazine hydrochloride is a pink solid powder with an odor and a pungent smell at room temperature. It is a highly toxic substance and is mainly used as an organic intermediate in the dye, pharmaceutical and pesticide industries. For example, it can Produces naphthol AS-G, drug antipyrine, cationic dyes, developers, etc.
Synthesis【1】
For some small and medium-sized enterprises, due to limitations of production process conditions, the purity of the products is not high, so that the products are mainly used in the pesticide industry. And often due to improper determination of certain synthesis process conditions, abnormal reactions occur, which affects product yield and quality to a certain extent.
1. Synthesis process
The production of phenylhydrazine hydrochloride uses aniline as the main raw material, and carries out diazotization reaction with sodium nitrite at low temperature in acidic medium to generate diazonium salt-diazobenzene chloride, and then uses ammonium bisulfite and carbonic acid. Ammonium hydride is reduced to form sodium diazobenzene disulfonate, which is then separated by acid to form phenylhydrazine hydrochloric acid. That is, it mainly includes the two-step process of diazotization of aniline and diazobenzene chloride.
The process flow used is shown in the attached picture:
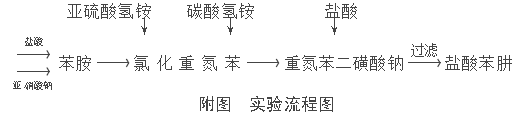
2. Chemical reaction mechanism
2.1 Diazotization reaction of aniline
(1)Ar—NH2 + HCl→ Ar—NH2·HCl
(2)NaNO2 + HCl→ HNO2 + NaCl
(3)Ar—NH2 + 2HCl+ NaNO2 → Ar—N—NCl + NaCl + 2H2O
2.2 Diazonium salt reduction reaction
(1)Ar—N—NCl + NH4HSO3 + NH4HCO3 → Ar—N—NSO3NH4 + NH4Cl + CO2 ↑+H2O
(2)Ar—N—NSO3NH4 + NH4HSO3 → Ar—N—NHSO3NH4
SO3NH4
(3)Ar—N—NHSO3NH4 + H2O → Ar—NH—NHSO3NH4 + NH4HSO4
∣
SO3NH4
2.3 Acid precipitation to form salt
(1)Ar—NH—NHSO3NH4 + HCl + 2H → Ar—NHNH2·HCl + NH4HSO3
(2)NH4HSO3 + HCl → NH4Cl + H2O + SO2 ↑
3. Factors affecting the preparation of diazobenzene chloride
In the first reaction unit in the synthesis of phenylhydrazine, that is, in the process of preparing diazobenzene chloride by diazotization of aniline, certain reaction conditions are often improperly determined, causing abnormal phenomena in the reaction, resulting in product yield and quality problems. bring certain impact. Therefore, determining the proportion of raw material input, reaction temperature, and sodium nitrite solution addition rate are important influencing factors. Through orthogonal experiments, the best reaction conditions are found as follows:

3.1 Effect of the dosage of hydrochloric acid in diazotization reaction
Because there are electron groups on aniline, it is sufficiently alkaline. During diazotization, the amount of hydrochloric acid must be at least three equivalents. If the amount of acid is insufficient and free amines are present (aromatic amines are weak bases, and their salts are easy to decompose), diazoamine compounds will be produced. Experiments have shown that when the amount of hydrochloric acid exceeds 2.3, the reaction liquid becomes transparent and the product yield is higher. When the amount of hydrochloric acid is small, the diazo liquid will become turbid. This is because the diazo reacts in a neutral or weakly acidic solution (pH 5 to 7).The reducing solution should be prepared in advance before the reaction reaches the end point. As soon as the diazotization reaction is completed, if it is checked that the end point has been reached, the reduction reaction should be carried out immediately. The test used cheap ammonium bisulfite and ammonium bisulfate as reducing agents, which reduced production costs. In the reduction reaction, the pH value of the prepared reducing solution is crucial and should be controlled between 6.2 and 6.7. Control the reduction reaction temperature. The optimal reduction temperature is between 80 ~ 85 ℃, and the time is about 60 ~ 70 minutes. After the diazo liquid reduction reaction, a small amount of zinc powder or iron powder must be added to reduce the trace amounts of benzene nitro compounds that may be present in the reactants to eliminate the variegation in the phenylhydrazine product and improve the purity of the product.
5. Study on acid precipitation reaction conditions
The diazobenzene disulfonic acid sodium salt generated after the reduction reaction of the diazonium salt reacts with water to form phenylhydrazine sodium sulfonate, which then reacts with hydrochloric acid to form phenylhydrazine hydrochloride. After adding the hydrochloric acid, continue to raise the temperature and keep it between 80 and 85°C and stir for 60 minutes. After cooling down, phenylhydrazine hydrochloride will precipitate. After filtering or drying, the product phenylhydrazine hydrochloride can be obtained.
Through the study of various important influencing factors in the synthesis experiment of phenylhydrazine hydrochloride, the optimal conditions for each stage of the reaction were found. The resulting product purity was 85.90% and the yield was 89.35%. Moreover, the diazotization reaction time is shortened, which not only shortens the operation time, but also reduces the possibility of side reactions, thereby ensuring the quality of the diazo liquid and improving the product yield. At the same time, low-priced ammonium bisulfite and ammonium bicarbonate are used as reducing agents, which further reduces production costs and achieves better economic benefits.
Pharmacokinetics【2】
Phenylhydrazine hydrochloride is an intermediate chemical raw material used in the synthesis of dyes and drugs. It is moderately toxic and can be absorbed through the respiratory tract, gastrointestinal tract, and skin. The oral LD50 in rats is 188 mg/kg, and the oral toxic dose in humans is approximately 0.2g. After phenylhydrazine hydrochloride enters the body, it mainly exerts its effect through phenylhydrazine. Phenylhydrazine is an oxidant that can oxidize hemoglobin in red blood cells into methemoglobin, and the molecules rearrange into methemochromogen, which further becomes irreversible hemochromogen. With the strong binding of band 3 protein to methemochromogen and the “propagation” of this interaction on the membrane, it eventually leads to the formation of Heinz bodies on the membrane. Due to the lack of flexibility and poor deformability of the membrane at this time, Therefore, it is easy to hemolyze in the body.
Poisoning【2】
Acute poisoning by phenylhydrazine mainly damages the hematopoietic system and the central nervous system. Those with mild poisoning may have symptoms such as dizziness, headache, weakness, pale complexion, loss of appetite or abdominal pain and diarrhea; severe poisoning may cause severe headache, tinnitus, dizziness, Shortness of breath, difficulty breathing, convulsions, tremors and even ataxia and unconsciousness; severe cases may have cyanosis, jaundice and leukopenia, and hemolytic anemia, hyperbilirubinemia, toxic liver disease and kidney damage may occur.
Clinical diagnosis is based on medical history, clinical manifestations and onset characteristics, and the diagnosis is usually without difficulty. However, phenylhydrazine poisoning is rare clinically, and there is a lack of understanding of its clinical characteristics and treatment. In addition to general treatments such as cleaning the local area with water, light acetic acid or ethanol, and inhaling oxygen, acute phenylhydrazine poisoning can be treated with specific treatments such as glucocorticoids, 1% melan, 20% sodium thiosulfate, and vitamin C. Clinical recovery time It takes about 2 to 3 weeks.
References
[1] Zhang Ru, Wu Yinzhi, Zheng Dianmo, Synthesis of Phenylhydrazine Hydrochloride, Journal of Nanchang University (Science Edition), 2002.12, page 395
[2] Dai Lingyun, Wu Dejun, 4 cases of phenylhydrazine hydrochloride poisoning, Chinese Journal of Emergency Medicine, 2003.05, page 347



 微信扫一扫打赏
微信扫一扫打赏
