Background and overview[1][2]
2-Chloro-3-fluorobenzoic acid is mainly used as an intermediate in pharmaceutical synthesis. If 2-chloro-3-fluorobenzoic acid is inhaled, move the patient to fresh air; if the skin comes into contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eyes If exposed to sunlight, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
Apply[1-2]
2-Chloro-3-fluorobenzoic acid is mainly used as an intermediate in pharmaceutical synthesis. Examples of its applications are as follows:
1. Used to prepare a poly-substituted benzoic acid compound, 6-amino-2-chloro-3-fluoro-benzoic acid. The specific steps of the preparation method are as follows: 1) At 10°C, 20g of compound 1 (2 -Chloro-3-fluoro-benzoic acid) was slowly added to 60 ml of concentrated sulfuric acid. After the system was completely dissolved, the system was cooled down; when T = 0°C, fuming nitric acid was added dropwise to the system. The dropping time is 10 minutes. After the dropwise addition is completed, the system returns to room temperature and stirs for about 4 hours; LC-MS shows that the raw material reaction is complete. Pour the system into 200 ml of ice water and immediately precipitate the solid. Filter and wash the filter cake with ice water. After drying, prepare under medium pressure. 5.1 g of white solid (compound 2) was obtained, yield = 20%. (2) Dissolve 4g of compound 2 in methanol, add Raney nickel, replace the system with 0.1MP H2, and stir at room temperature for 21 hours; LCMS shows that the raw material reaction is complete, the system is slightly concentrated to a small amount of solvent remaining, and the residue is treated with NaOH The solution was adjusted to pH = about 10, and the system was suction-filtered through diatomaceous earth. Concentrated hydrochloric acid was added dropwise to the solution until no more solid precipitated. The solution was filtered and dried to obtain 2 g of yellow solid (compound 3), yield = 58%.
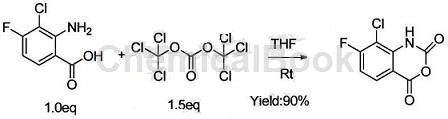
2. Preparation of 5-amino-7-fluoroquinoline. Quinoline compounds are a very important class of compounds among nitrogen heterocyclic alkaloids, with significant physiological activity and broad application prospects. The latest research shows that small molecules with aminoquinoline structures can serve as brain-penetrating selective JNK inhibitors, and the aminoquinoline structure can improve the pharmacokinetic properties of drugs used to prevent neurodegenerative diseases. In addition, this type of compound can also serve as a sub-trace inhibitor of the new NAD-hydrolase CD38, effectively improving geriatric diseases and obesity. Because of the potential effects of aminoquinoline compounds, medicinal chemists have conducted extensive research on their medicinal properties, and usually introduce fluorine atoms to improve their medicinal properties. Among them, 5-amino-7-fluoroquinoline is an important compound among this type of fluorine-containing aminoquinoline compounds. However, there are currently few reports on the synthesis of this compound and the yield is low. In some experiments, simple and easily available 2-chloro-3-fluorobenzoic acid was used as the starting material, and the title compound was obtained through a 6-step reaction of substitution, Hofmann degradation, ring closure, nitration, iron powder reduction, and hydrogenation and dechlorination of 8-chloride. . This process route has low cost, easy operation, and a total yield of up to 43%, making it suitable for industrial production.
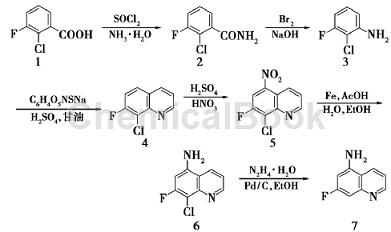
The specific steps are as follows:
1) Synthesis of 2-chloro-3-fluorobenzamide (2): In a 100 mL three-neck reaction flask, add 35 mL of thionyl chloride, add 5.00 g (28.6 mmol) of compound 1 in batches, and add After completion, the mixture was heated to reflux at 80°C for 4 h. Concentrate to remove thionyl chloride, and dissolve the residue in 15 mL of methylene chloride for later use. In another 250 mL three-necked flask, add 50 mL ammonia water, bathe in ice, slowly add the reserve solution dropwise, and stir for 30 minutes after adding. Filter with suction, rinse with 5 mL of water, and dry to obtain 4.90 g of white powder, with a yield of 98%.
2) Synthesis of 2-chloro-3-fluoroaniline (3): In a 1 L three-neck reaction flask, add 540 mL (2 mol /L)
Sodium hydroxide, cool down to 0°C, slowly add 34.8 g (218mmol) Br2 dropwise, complete the addition in 30 minutes, stir at 0°C for 30 minutes. Add 30.0 g (173 mmol) of compound 2 in batches and react at 0°C for 4 h and 60°C for 9 h. Cool to room temperature, remove the water phase, adjust the precipitate to pH 2 with concentrated hydrochloric acid to form a salt, wash with 30 mL of eluent (V (petroleum ether): V (ethyl acetate) = 5:1), stir for 30 minutes, and pump Filter, rinse with 15mL petroleum ether, adjust the filter cake to pH 7 with saturated aqueous sodium bicarbonate solution, extract with ethyl acetate (300 mL×3), combine the organic phases, dry over anhydrous sodium sulfate, and concentrate to obtain 19.8 g of brown-red liquid. The yield is 79%.
3) Synthesis of 8-chloro-7-fluoroquinoline (4): In a 500 mL three-neck reaction flask, add 12.0 g ( 82.4
mmol) Compound 3, 120 mL (70%) H2SO4, 27.8 g (124mmol) sodium m-nitrobenzene sulfonate, heat to 100 ℃, add 15.0g (163 mmol) glycerol dropwise, and react at 160 ℃ for 6 hours. Cool to room temperature, pour into ice to quench the reaction, and filter through diatomaceous earth. The filtrate was adjusted to pH 7 with 10% sodium hydroxide, and the solid was washed out and filtered with suction. The filter cake was dissolved in ethyl acetate and filtered again with suction. The filtrate was concentrated to obtain 12.0 g of purple-black solid, with a yield of 80%.
4) Synthesis of 5-nitro-8-chloro-7-fluoroquinoline (5): In a 1L three-neck reaction flask, add 145 mL of concentrated sulfuric acid, and add 42.0 g (231 mmol) of compound 4 in batches. Stir evenly, cool the ice bath to 0°C, slowly add 66 mL fuming nitric acid dropwise, remove the ice bath to room temperature, and react at 80°C for 4 hours. Cool, pour into ice to quench the reaction, and filter with suction to obtain 40.8 g of light yellow solid, with a yield of 78%.
5) Synthesis of 5-amino-8-chloro-7-fluoroquinoline (6): In a 2L three-neck reaction flask, add 71.0 g (1.27mol) iron powder, 600 mL ethanol, 800 mL water, 21.2 g (353mmol) acetic acid, heated to reflux, add 40.0 g (177mmol) chemical in batchesAdd compound 5 and reflux for 10 minutes after adding. Add 800 mL of ethanol, heat to reflux, filter through diatomaceous earth while hot, rinse with 500 mL of hot ethanol, concentrate the filtrate, and purify by column chromatography (V (petroleum ether): V (ethyl acetate) = 4: 1) to obtain 33.2 g dark yellow solid, yield 95%.
6) Synthesis of 5-amino-7-fluoroquinoline (7): In a 1 L three-neck reaction flask, add 29.0 g ( 148
mmol) Compound 6, 7.90 g (73.8 mmol, 10%) palladium on carbon, 145 mL hydrazine hydrate, 580 mL absolute ethanol, reflux reaction at 95 °C for 4 h. Cool, suction filtrate, concentrate the filtrate, and purify by column chromatography (V (petroleum ether): V (ethyl acetate) = 4:1) to obtain 22.0 g of white solid, yield 92%, m.p. 136.3~136.6℃.
Main reference materials
[1] CN201410230998.4 A multi-substituted benzoic acid compound and its preparation method
[2] Zhao Xiaoying, Xu Weiliang, Yin Xiaoxing, et al. Synthesis of 5-amino-7-fluoroquinoline[J]. Chemical Reagents, 2018, 40(2): 192-194.



 微信扫一扫打赏
微信扫一扫打赏
