Background [1][2]
β-Bromophenylethane is an important intermediate for the synthesis of high-efficiency brominated flame retardants, polybrominated styrene, and is also commonly used in the preparation of organic synthesis intermediates. For example, β-bromophenylethane can be used to prepare the pranlukast intermediate 4-(4-phenylbutoxy)benzoic acid. As an anti-asthmatic drug, pranlukast was first successfully developed by Japan’s Ono Company and launched in Japan in 1995. In 1999, it was discovered that it could treat allergic rhinitis. Pranlukast is a leukotriene C4/D4 receptor antagonist (lTRAs) with extremely low toxicity. It can selectively inhibit the activity of leukotriene polypeptide in airway smooth muscle and has almost no effect on arachidonic acid metabolism enzymes. , while having no antagonistic effect on acetylcholine, 5-hydroxytryptamine, etc., and significantly inhibiting lTC4, lTD4, lTE4, etc.
Research shows that in clinical application, pranlukast has good therapeutic effects not only on atopic asthma, but also on other types of bronchial asthma such as mixed, infectious, paroxysmal, chronic and non-seasonal asthma. , is one of the hot spots in asthma drug development and research in recent years. There are two synthesis pathways for β-bromophenylethane: one is the anti-Markovsky rule addition of hydrogen bromide and styrene under the stimulation of ultraviolet and x-ray irradiation; the other is the substitution of phenylethyl alcohol with a brominating agent to obtain the target product. At present, the traditional production method is mostly produced by the reaction of phenylethyl alcohol and hydrogen bromide (or hydrobromic acid).
Synthetic route [2]
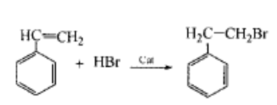
Using cheap and easily available styrene as raw material, in the presence of an initiator, hydrogen bromide gas is passed into the alkane solution of styrene to perform anti-horse addition to obtain β-bromophenylene ethane, yield Greater than 95%, purity greater than 99%. Compared with traditional processes, this method has the advantages of cheap and easily available raw materials, easy separation of products, simple production process, low cost, and high product quality. The specific steps are as follows: pre-add a certain amount of styrene alkane solution into a four-neck reaction bottle, add styrene and alkane at a volume ratio of 1:5, then add an appropriate amount of catalyst, and heat in an oil bath. Stir thoroughly and heat to reaction temperature (80~90℃), then add hydrogen bromide gas.
Let it bubble evenly in the reaction solution, and form a saturated solution of hydrogen bromide in the reaction system. In this medium, styrene and hydrogen bromide undergo a free radical addition reaction to generate β-bromophenyl alkyl. After the reaction is completed, the product is distilled under reduced pressure, the solvent is recovered, and the clear and transparent fraction obtained is the target product.
Purpose[1][3][4]
β-Bromophenylethane is an important intermediate for the synthesis of high-efficiency brominated flame retardants, polybrominated styrene, and is also commonly used in the preparation of organic synthesis intermediates. Examples of its application are as follows:
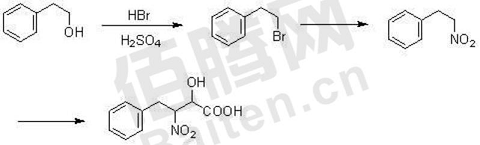
1. Preparation of 2-nitro-3-phenyl-1-propanol.
2 Nitro 3 Phenyl 1 Propanol is an important class of pharmaceutical intermediates, which plays an important role in the preparation of fully synthetic antibacterial agents (R) 4 Benzyl 2 oxazolidinones role. In 1960, nitrostyrene was chosen as the starting material. After reducing the double bond, hydroxymethyl was introduced to synthesize 2 nitro 3 phenyl 1 propanol. However, when the double bond was reduced, the nitro group was also reduced, resulting in the occurrence of side effects.
Use tertiary amine as the α position of Lewis base to activate nitroalkane, select paraformaldehyde as raw material, first nitrate β-bromophenylene ethane, remove the proton and then introduce hydroxymethyl to prepare 2 nitro groups 3 phenyl 1 propanol has low production cost and overcomes the shortcomings of harsh reaction conditions and complicated synthesis steps. Specifically, follow the following steps: (1) Perform nitration reaction between β-bromophenylethane and NaNO2. During the reaction, the temperature is controlled at 0-10°C and the reaction time is controlled at 3-5h; (2) Triethylamine is added for decontamination. protons, and then add paraformaldehyde for reaction. The reaction temperature is controlled at 20-40°C, the reaction time is 3-5h, and purified by recrystallization from toluene to obtain 2 nitro 3 phenyl 1 propyl alcohol.
2. Preparation of polybrominated styrene and its monomers.
Polybrominated styrene is an additive polymer brominated flame retardant with a bromine content of up to 69%. It has the advantages of good thermal stability, high decomposition temperature, low toxicity and good flame retardant effect. It has been widely used It is used for flame retardant treatment of thermosetting plastics such as PET, PBT, polyphenylene ether, nylon 66, nylon 6, polyimide, syndiotactic polystyrene, unsaturated polyester and epoxy resin. There are studies using β-bromophenylene ethane as raw material, and in the presence of a catalyst, a mixture of polybrominated β-bromophenylene ethane is obtained by bromination with a brominating agent.
The mixture is refluxed under an alcoholic sodium hydroxide solution to remove hydrogen bromide to obtain polybrominated styrene monomer. Polybrominated styrene can be obtained by bulk, solution and suspension polymerization of the monomer mixture. The advantage of this method is that compared with brominated polystyrene, it has better thermal stability and is less likely to change color at high temperatures. The prepared polybrominated styrene has a controllable bromine content (up to 69%) and a controllable molecular weight. It can be used for flame retardant of PET, PBT, nylon and other resins.
3. Preparation of pranlukast intermediate 4-(4-phenylbutoxy)benzoic acid.
Using β-bromophenylene ethane as the starting material, 4-(4-phenylbutoxy)benzene is obtained through Grignard reaction, addition of ethylene oxide, mesylation, substitution and hydrolysis. Formic acid. This method has the characteristics of cheap and easily available raw materials, short process route, good product purity, high yield and low cost.Integrate industrial production.
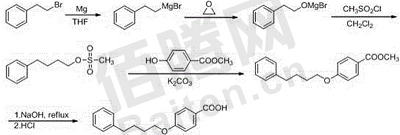
The above process route includes the following steps:
a. Synthesis of 4-phenylbutoxysulfonate: Add magnesium powder, iodine granules and part of the mixture of anhydrous tetrahydrofuran and β-bromophenylethane to a three-necked flask equipped with a reflux condenser. , initiated the reaction at 25°C. After the reaction is initiated, the remaining β-bromophenylene ethane and anhydrous tetrahydrofuran mixture is slowly added dropwise under stirring, and the dropping speed is controlled to keep the system slightly boiling. After the mixture is added dropwise, continue heating and refluxing for 30 minutes, then stop heating and cool to room temperature. Place the system in an ice-salt bath, control the temperature at 0~10°C, and introduce 1~1.2 equivalents of ethylene oxide through the catheter.
After all the ethylene oxide has been introduced, remove the ice bath and heat to reflux for 1 hour. The solvent tetrahydrofuran was evaporated and cooled to room temperature. Add methylene chloride to the above system to dissolve, add the sulfonylation reagent dropwise in an ice bath, control the temperature not to exceed 10°C, complete the dropwise addition, and keep the reaction for 3 hours. At the end of the reaction, add water to the system to quench the reaction, stir, let stand and separate layers, extract with dichloromethane, and rotary evaporate the solvent to obtain 4-phenylbutoxysulfonate;
b. Synthesis of 4-(4-phenylbutoxy)benzoic acid methyl ester: Add 4-phenylbutoxysulfonate, methyl p-hydroxybenzoate and potassium carbonate to toluene, stir Heat and reflux for 11 to 15 hours. After the reaction is completed, cool to room temperature, add water and stir for 10 minutes, let it stand to separate the organic layer, and remove toluene under reduced pressure to obtain 4-(4-phenylbutoxy)benzoic acid methyl ester;
c. Synthesis of 4-(4-phenylbutoxy)benzoic acid: Add the above product and 25% NaOH solution to ethanol, and heat to reflux and hydrolyze for 3 to 4 hours. After the reaction is completed, cool down, remove ethanol under reduced pressure, acidify to pH = 2, and precipitate a white solid, which is filtered and dried to obtain the product 4-(4-phenylbutoxy)benzoic acid.
Main reference materials
[1] CN201410619968.2 New synthesis route of pranlukast intermediate 4-(4-phenylbutoxy)benzoic acid
[2] Yan Xiaohong, Li Shanqing, Song Ningning, et al. Research on the synthesis process of β-bromophenylene ethane [J]. Fine Chemical Intermediates, 2013, 43(3): 45-47.
[3] CN201110346763.8 A method for preparing 2-nitro-3-phenyl-1-propanol
[4] CN200710010654.2 Preparation method of polybrominated styrene and its monomer



 微信扫一扫打赏
微信扫一扫打赏
