Overview【1】
Methoxyacetophenone has a strong and lasting hay aroma, coumarin-like aroma, floral and hawthorn aroma. The appearance is crystalline. p-Methoxyacetophenone can be used in daily chemical fragrance formulas. Since it is relatively stable in alkaline medium, it is especially suitable for use in soap and synthetic detergent fragrance formulas. The dosage in fragrance formulas is within 5%.
Purpose【2】
P-Methoxyacetophenone is an indispensable raw material for fragrances such as mimosa and hawthorn. It is often used in high-end cosmetics and soap flavors; it can also be used as fruit food flavor.
Synthesis【3】【4】【5】
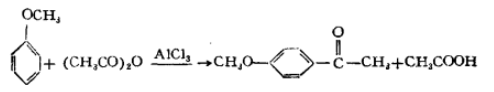
1. Anisole interacts with acetic anhydride or acetyl chloride in the presence of anhydrous aluminum trichloride, and is produced through acetylation reaction. The reaction formula is as follows:
Process flow:
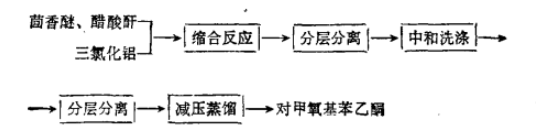
2. Catalytic synthesis of p-methoxyacetophenone by gallium phosphotungstate
P-methoxyacetophenone (p- MOAP).
Preparation of catalyst:
Prepare GaPW12O40 catalyst according to the method reported in the literature: weigh a certain amount of H3PW12O40 and gallium nitrate, dissolve them in a certain amount of distilled water respectively, add the gallium nitrate solution dropwise to the H3PW12O40 solution at room temperature; after the dropwise addition is completed, Continue to stir for a certain period of time, age at 80°C for 1 h, then slowly evaporate the excess water, and dry at 120°C overnight to obtain the GaPW12O40 catalyst. The catalyst was calcined at 300 °C for 2 h before use.
Preparation of InPW12O40 catalyst: It is prepared by reacting H3PW12O40 with indium nitrate. The preparation method is the same as above.
Synthesis and purification of p-MOAP:
Add an appropriate amount of anisole and catalyst into a 100 mL three-neck round-bottom flask, install a reflux device, stir magnetically, heat the oil bath to a certain temperature, use a constant pressure funnel to slowly drip in an appropriate amount of acetic anhydride, and conduct a constant temperature reaction. After a certain period of time, cool to room temperature. Calculate the product yield based on the mass of the reaction solution and the amount of acetic anhydride. After the above reaction liquid is separated from the catalyst, it is washed with alkali, washed with water, dried with anhydrous magnesium sulfate, and distilled under reduced pressure to collect the 152-154°C (3.5 kPa) fraction to obtain the p-MOAP product.
The optimal conditions for the synthesis of p-MOAP are: 0.1 g of GaPW12O40 catalyst pretreated at 300 ℃, 20 mmol of acetic anhydride, n (anisole): n (acetic anhydride) = 5, reaction temperature 90 ℃ , the reaction time is 30 minutes, the conversion rate of acetic anhydride reaches 98.49%, the selectivity of p-MOAP reaches 94.91% and the purity is high.
The GaPW12O40 catalyst is extremely prone to coking and carbon deposition during the reaction process, which seriously hinders the reuse of the catalyst, so its stability needs to be further improved.
3. Iodine-catalyzed synthesis of p-methoxyacetophenone
Using iodine as a catalyst, p-methoxyacetophenone is synthesized by acylation reaction of anisole and acetic anhydride.
In the reaction flask, add the catalyst, anisole and acetic anhydride, install a reflux condenser, and use a magnetic stirrer.Stir. Control a certain reaction temperature through a water bath or oil bath, react at a constant temperature for a certain period of time and then cool. Take a small sample for quantitative gas chromatography analysis. Add water to the filtrate to hydrolyze unreacted acetic anhydride, let it stand and separate the layers. Wash the organic layer with sodium hydroxide solution until it is nearly neutral. The organic layer obtained after liquid separation is dried with anhydrous magnesium sulfate. First, distill it under normal pressure to recover benzene. Light fractions such as methyl ether are collected, and then the fraction at 152 ~ 154 ℃ /3.5 kPa is collected under reduced pressure, which is the reaction product p-methoxyacetophenone.
Based on the acylation reaction mechanism, it is speculated that the mechanism of iodine-catalyzed acylation reaction is as follows:
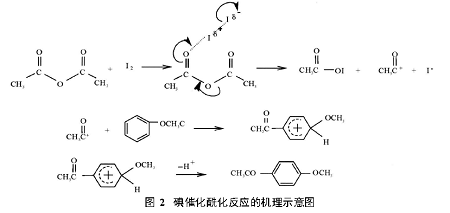
The optimal reaction conditions are: 0.1 mol of anisole, the molar ratio of anisole to acetic anhydride is 1: 1.5, the optimal mass of the catalyst is 1 g, the reaction time is 1.5 h, and the reaction temperature is 100 °C. The product yield reached 52.9%. The catalytic effects of different types of catalysts on the acylation reaction of anisole and acetic anhydride were compared. The results show that the catalytic efficiency of iodine is much higher than that of heteropoly acids and zeolite molecular sieves, and it is a highly efficient catalyst for acylation reactions.
4. Synthesis of p-methoxyacetophenone catalyzed by heteropolyacids
Using heteropoly acid as a catalyst, p-methoxyacetophenone is synthesized by acylation reaction of anisole and acetic anhydride.
Catalyst pretreatment and preparation of supported heteropolyacids:
Put the heteropoly acid into a controllable oven and dry to a certain temperature, and dry at a constant temperature for 1.5 h. Loaded H3 [PW12 O40] was prepared by excess impregnation method. Mix and stir a certain amount of carrier with the aqueous solution of H3 [PW12O40], keep it in a water bath at a certain temperature for 14 hours, then evaporate it to dryness in the water bath, then put it into a controllable oven and dry it at 150°C for 2 hours to get the load. H3[PW12O40].
Friedel-Crafts acylation reaction of anisole and acetic anhydride:
In the reaction flask, add the catalyst, anisole and acetic anhydride, install a reflux condenser, and stir with a magnetic stirrer. Control a certain reaction temperature through a water bath or oil bath, react at a constant temperature for a certain period of time and then cool. Chloroform was added to precipitate the heteropoly acid, and the catalyst was filtered out. Add water to the filtrate to hydrolyze unreacted acetic anhydride, let it stand and separate the layers, wash the organic layer with sodium hydroxide solution until it is nearly neutral, dry the organic layer obtained after liquid separation with anhydrous magnesium sulfate, distill it under normal pressure first, and recover chloroform. , anisole and other light fractions, and then collect the fractions at 152~154°C under a pressure of 3.5Kpa under reduced pressure, which is the reaction product p-methoxyacetophenone.
The optimal reaction conditions are: the catalyst needs to be pretreated, the temperature is 150°C, the molar ratio of anisole and acetic anhydride is 1:1.5, the optimal mass ratio of anisole and catalyst is 11, the reaction The time was 4h, the reaction temperature was 100°C, and the product yield reached 52.9%. Under optimal conditions, the catalytic effects of different heteropolyacids and loaded heteropolyacids on the acylation reaction of anisole and acetic anhydride were investigated. As the strongest acid among heteropolyacids, phosphotungstic acid has the best catalytic activity.
References
[1] Compiled by Liu Shuwen, Synthetic Flavor Technology Manual, People’s Music Publishing House, 2009.1, page 217
[2] Beijing Daily Chemical Industry Society, Chemical Products Manual Daily Chemical Products, Chemical Industry Press, May 1989, 1st edition, page 345
[3] Li Guixian, Liu Zhenzhen, Cao Yanwei, Li Yu, Synthesis of p-methoxyacetophenone catalyzed by gallium phosphotungstate, Petrochemical Industry, 2015.12, page 1476
[4] Shi Zhengbao, Qin Fang, Chen Ping, Iodine-catalyzed synthesis of p-methoxyacetophenone, Flavors and Fragrances Cosmetics, 2009.12, page 22
[5] Xu Mei, Li Hongpeng, Chen Ping, Yu Tingyun, Li Xiaoou, Synthesis of p-methoxyacetophenone catalyzed by heteropoly acids, Flavors, Fragrances and Cosmetics, 2006.06, page 10



 微信扫一扫打赏
微信扫一扫打赏
