Background and overview[1][2][3]
2-Phenyl-1-propene, also known as α-methylstyrene (abbreviated as a-MS or AMS) or phenylisopropylene, is a by-product of the cumene production of phenol acetone, generally per ton The by-product of phenol is 0.045t α-MS. 2-Phenyl-1-propene is a colorless liquid with a pungent odor. The molecule contains a benzene ring and an alkenyl substituent on the benzene ring. It is prone to polymerization when heated. 2-Phenyl-1-propene can be used in the production of coatings, plasticizers, and as a solvent in organic synthesis.
Synthesis method[4][5]
Synthetic path 1:
Use cumene as the starting material, oxidize the cumene, and use the cumene hydroperoxide stream. Without concentrating the cumene hydroperoxide stream, at least a part of it is separated and selectively Hydrogenation reaction is carried out under a precious metal catalyst to prepare cumyl alcohol, the reactant containing the cumene alcohol is concentrated, and dehydration reaction is performed in an acidic environment to prepare 2-phenyl-1-propene.
Synthesis path 2:
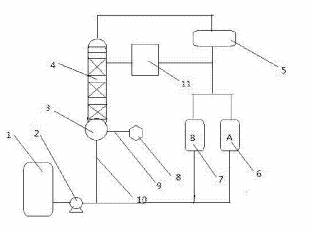
Recover 2-phenyl-1-propene from phenol waste liquid. The device diagram is shown below. Add the phenol residual liquid into the raw material storage tank, and transport the phenol residual liquid to the tower kettle through the pipeline through the power of the power pump. Under the heating effect of the heater, the phenol residual liquid in the tower kettle is heated to 170°C to separate isopropyl. Benzene and 2-phenyl-1-propene are transported to the condenser through the reaction tower, liquefied under the action of the condenser, transported to the reflux tank through the pipeline, and transported to the reaction tower and tank A in a ratio of 5:1 respectively. , continue to heat to 250°C to separate phenol and acetophenone, transport them to the condenser through the reaction tower, liquefy them under the action of the condenser, transport them to the reflux tank through the pipeline, and transport them to the reaction in a ratio of 5:1 respectively. Tower and tank B. The cumene and 2-phenyl-1-propene in tank A are transported to the tower kettle through pipelines. Under the action of the heater, the components in the tower kettle are heated until 2-phenyl-1-propene is separated. It is transported to the condenser through the reaction tower, liquefied under the action of the condenser, transported to the reflux tank through the pipeline, and transported to the reaction tower and tank A at a ratio of 5:1 respectively, and the product is collected from tank A.
Application fields[1][2][3][6][7]
1. Organic synthesis intermediates
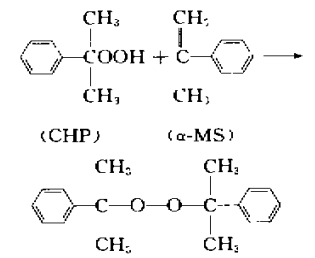
α-MS is a basic raw material for organic synthesis and can be used to synthesize cumene, dicumyl peroxide, p-cumylphenol, etc. The hydrogenation of α-MS to produce cumene was industrialized in 1980, using a palladium/aluminum catalyst. The reaction was carried out in a trickle bed reactor. The conversion rate of α-MS was greater than 99%, and the by-products were very few. Therefore, the cumene Can be returned directly to the phenol acetone production line. The synthesis of dicumyl peroxide (DCP) by α-MS and cumene hydroperoxide (CHP) is another process route for DCP synthesis. It uses phenol as a catalyst and a reaction temperature of 15 to 45°C to synthesize DCP in one step. Compared with the traditional benzyl alcohol process, the reduction, water washing and concentration steps of preparing benzyl alcohol can be omitted. The reaction formula is shown in the figure below. P-cumylphenol, synthesized from α-MS and phenol under the action of acid catalyst, is a raw material for the synthesis of thermoplastics and polycarbonate.
2. Copolymerized monomer
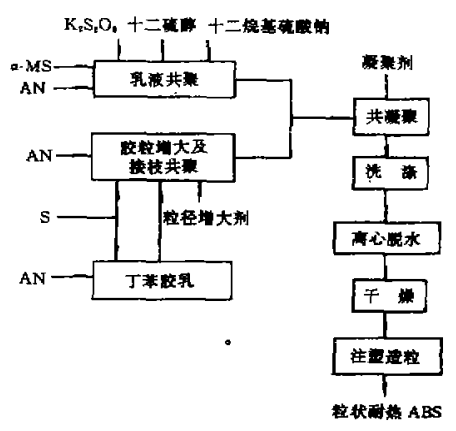
α-MS can be used as a resin modifier to copolymerize with acrylonitrile, methyl methacrylate, maleic anhydride, etc. to produce copolymer resins with different characteristics. In the 1970s, there were dozens of α-MS The emergence of copolymers has penetrated into rubber, plastics, adhesives, coatings and other fields. Among them, the use of α-MS for heat-resistant modification of ABS resin is the largest consumption area. 45% of α-MS on the market is used to synthesize ABS resin. The most typical method to improve the heat resistance and rigidity of ABS resin is to replace styrene with α-MS. α-MS-AN copolymer can be miscible with ABs resin in any ratio. Through the measurement of glass transition temperature and transmission electron microscope observation, it was proved that the prepared heat-resistant ABS resin has a microscopic two-phase structure, in which α-MS-AN copolymer and SAN in the ABS graft constitute the continuous phase of the resin, and the rubber particles are dispersed Phase, α-MS-AN copolymer provides its heat resistance and rigidity, and ABS provides toughness. Compared with general-grade ABS resin, the heat distortion temperature of modified ABS resin is increased by 10 to 15°C. The process flow for preparing heat-resistant ABS resin is as follows.
3. Synthetic fragrances
Using α-MS as the starting material, through condensation reaction, hydroxyisopropylation reaction, chloromethyl etherification reaction and cyclization reaction, galaxanthin (galmusk) with excellent performance and currently the most widely used can be produced. Its trade name is Galaxolide). The synthesis route of Galamusk is as follows. Its intermediate product hexamethylindanol is also a valuable spice after being refined. It has a strong and long-lasting aroma of art, amber, and musk, and can be directly used as a blending essence for soaps, detergents, talcum powder, etc. preparation. Pentamethylindane can also undergo Friedel-Crafts reaction under the catalysis of aluminum trichloride, using acid chloride as the acylating agent to produce woody, fruity, amber, and musk-type fragrances. Pentamethylindan can also be used to synthesize many new varieties such as indanol, indanone, and indanethers with fragrance value. In addition to galaxol, 2-phenyl-1-propene can also be used to synthesize spices such as cashmerone (with a strong, long-lasting and sweet musky woody aroma) and solanol (with an elegant rose aroma).
4. Synthetic oligomers
Using a solid acid that can generate active cations as a catalyst, α-MS can polymerize through the mechanism of ionic polymerization to obtain low molecular weight polymers. The degree of polymerization is closely related to the reaction temperature. α-MS oligomer can significantly improve the rheological properties of plastics and significantly reduce the melt viscosity of polyolefin and polyvinyl chloride resins. It has been widely used as a plastic hope-increasing agent. Linear dimers can be used as cross-linking aids in the production of cross-linked polyethylene. In the polymerization reactions of acrylic coatings, adhesives, ABS, styrene, vinyl chloride, chloroprene and styrene-butadiene rubber, linear dimers serve as Used as a molecular weight regulator or chain transfer agent, its function is similar to that of dodecyl mercaptan, which can replace mercaptan molecular weight regulators and does not have the unpleasant smell of alkali alcohol substances. In lubricating oil manufacturing, linear dimers can increase the friction coefficient of lubricating oils, and hexamers can be used as viscosity modifiers for petroleum lubricating oils. Linear dimers can also be used as excellent fillers for various air cleaners, deodorants, soaps, detergents, shampoos, etc.
Main reference materials
[1] Yang Xiaohong. Comprehensive utilization of α-methylstyrene[J]. Gaoqiao Petrochemical, 2000, 15(6): 37-39.
[2] Ye Anyu. Developing the uses and market of α-methylstyrene[J]. Petrochemical Technology, 1994 (3): 134-138.
[3] Mei Laibao, Zhou Zhuohua. Utilization of α-methylstyrene[J]. Liaoning Chemical Industry, 1994 (5): 18-20.
[4] He Chengbai, Yu Xijun, Cao Dongxuan, preparation method of 2-phenyl-1-propene, CN 201180037274, application date 2011-10-10
[5] Dong Qilong, Fan Changsheng, Li Xuebin, a device for recovering 2-phenyl-1-propene from phenol residue, CN 201720343796, application date 2017-04-04
[6] Gu Jintao, Zhu Zhongnan. Research on the synthesis process of dicumyl peroxide from α-methylstyrene[J]. Chemistry World, 2000, 41(10): 529-532.
[7] Guo Xiuchun. Research on improving the properties of ABS resin with 2-phenyl-1-propylene-acrylonitrile copolymer[J]. Petrochemical Industry, 1989, 18(4): 220-226.



 微信扫一扫打赏
微信扫一扫打赏
