[Background and Overview][1][2]
SDHI (succinate dehydrogenase inhibitors) fungicides act on protein complex II, affecting the electron transfer system of the respiratory chain of pathogenic bacteria, hindering their energy metabolism, inhibiting the growth of pathogenic bacteria, and causing their death, thereby achieving the purpose of preventing and treating diseases. Purpose. It has a wide control spectrum, strong efficacy, long-lasting effect, and significant yield increase effect. In 2015, the global sales of SDHI fungicides were US$1.576 billion, of which triflufenac sales were US$390 million, accounting for 24.7% of the sales of SDHI fungicides.
Fluxapyroxad (trade name: Xemium) is an SDHI fungicide launched by BASF in 2012. It has both preventive and therapeutic effects and has excellent systemic conductivity activity. The market sales of triflufenac are quite strong. In 2012, the year it was launched, it achieved sales of nearly US$100 million (US$95 million); in 2013, the sales of triflufenac were 230 million. US dollars, soaring 142.11% year-on-year; in 2014, its global sales were US$285 million. The growth in sales was mainly driven by the Brazilian market. This was the first full year after the product was introduced to the Brazilian market in 2013; in 2015, Its global sales were US$390 million, driven by growth in South America.
So far, no such fungicide resistance issues have been reported. The main dosage forms of triflufenac are: emulsifiable concentrate (EC), suspending concentrate (SC) and seed treatment suspending concentrate (FS). The acute toxicity of triflufenac to mammals is very low. The acute oral LD50 value in rats (female) is >2 000 mg/kg, and the acute transdermal LD50 value in rats is >2 000 mg/kg. It is not irritating to the skin and eyes. Sex
[Physical and chemical properties and structure][1]
Triflufenacil, IUPAC name 3-(difluoromethyl)-1-methyl-N-(3′,4′,5′-trifluorobiphenyl-2-yl)pyrazole-4- Formamide, CAS name 3-(difluoromethyl)-1-methyl-N(3′,4′,5′-trifluoro[1,1′-biphenyl]-2-yl)-1H-pyra Azole-4-carboxamide. CAS registration number 907204-31-3, development code BAS 700 F, 5094351, molecular formula C18H12F5N 3O, relative molecular mass 381.3.
Pure triflufenacil is a white crystal with a melting point of 156.8°C, a relative density of 1.42 (room temperature), and a vapor pressure (25°C) > 8.1×10-9 Pa. Its solubility at room temperature (g/L, 20℃): acetone>250, acetonitrile 167.6±0.2, dichloromethane 146.1±0.3, ethyl acetate 123.3±0.2, methanol 53.4, toluene 20.0, n-octanol 4.69, n-heptane 0.106. Octanol/water partition coefficient (20℃) Kow logP is 3.08 (deionized water), 3.09 (pH4), 3.13 (pH7), 3.09 (pH9). When the pH value is 4 to 9, triflufenac is hydrolyzed stably, and there is no photolysis conversion phenomenon in natural water using artificial light (DT50 and quantum yield are not included).
[Main preparations][3]
The U.S. EPA has recommended approval of BASF’s new fungicide fluxapyroxad (trade name: Xemium). This approval decision covers two seed treatments. Two fungicides containing a single active ingredient for application to leaves and two combination formulations containing fluxapyroxad and pyraclostrobin (English trade name F500) for application to leaves. Application crops to be registered include fruit trees, vegetables and field crops. BASF intends to sell seed treatments containing a single active ingredient under the name Systiva, and market combination products containing fluxapyroxad/pyraclostrobin under the two names Merivon and Priaxor.
EPA has recommended approval of two seed treatments containing fluxapyroxad. Their code names are BAS 70002F (fluxapyroxad 334 g/L) and BAS 700 03F (fluxapyroxad 326 g/L), mainly for small particles. Grains, vegetables, cotton, soybeans, sunflowers and peanuts. BAS 700 02F is a suspension agent for on-farm and commercial seed treatment, while BAS 700 03F is a water-based seed treatment for commercial use only.
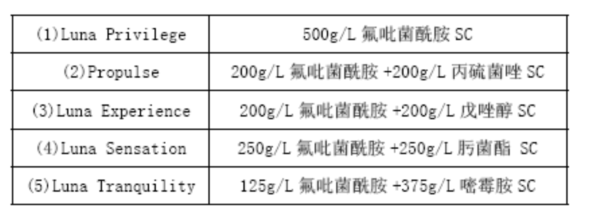
[Purpose][3]
Fluopyram is an SDHI fungicide developed by the company. It is mainly used to control powdery mildew, spot disease and ovum diseases in crops such as fruit trees, vegetables, cereals and grapes.
【Biological Activity】[1]
1. Indoor protective activity test against rice sheath blight: The test agent is 95% of the original drug triflufenac, and the test mass concentration is set to 100, 50, 25, 12.5, 6.25 mg/L, and there is no other facility. The blank control of the drug was repeated twice for each treatment. Select potted rice seedlings with uniform growth and the same leaf age. Use a crop sprayer to spray the leaves according to the above pesticide concentration. After spraying, place them in a fume hood to dry. After 24 hours, inoculate the rice sheath blight pathogen and use clamping. Inoculate using the bacterial block method. Each pot is connected with 3 0.25 cm2 rice sheath blight pathogen bacterial blocks, which are connected to the base of the rice seedlings. After inoculation, they were placed in an artificial climate greenhouse (temperature: 28°C day and 25°C night; relative humidity: 95%), moisturized and exposed to light for 7 days, and then investigated. During the investigation, the leaves of each pot with clamped bacterial blocks were investigated, and the degree of damage symptoms on the rice leaf sheaths and leaves was graded, and the control effect was calculated based on the disease index.
2. Indoor protection activities against cucumber powdery mildew.�Test: The test agent is 95% of the original drug of triflufenacil, and the test mass concentration is 200, 100, 50, 25, 12.5, 6.25 mg/L. Select potted cucumber seedlings that grow neatly and uniformly, spray them on a crop sprayer according to the above concentration of pesticides, and place them in a fume hood to dry after spraying. After 24 hours, inoculate cucumber powdery mildew spore suspension (5×106/mL). After inoculation, place it in the greenhouse for normal management (temperature: 23-28°C during the day and 18-20°C at night). The control effect will be investigated after 8 days of cultivation. Investigate the development of bacterial infection on each leaf. The prevention and treatment effect is calculated based on the disease index.
3. Indoor protective activity test against soybean rust. The test agent was 95% tritrifenpyramide original drug, and the test mass concentrations were 100, 25, 6.25, 1.56, and 0.39 mg/L. Select potted soybean seedlings (variety: Liaodou 10) that grow neatly and uniformly, cut off the growing points, keep 2 true leaves, spray them on the crop sprayer according to the set concentration, and set up a blank control without applying pesticides, 3 for each treatment times repeated. After spraying, place it in a fume hood to dry. After 24 hours, the soybean rust spore suspension (1×106/mL) was inoculated. After inoculation, it was placed in an artificial climate chamber (temperature: 25°C during the day and 20°C at night; relative humidity: 95% to 100%) for 1 d, and then moved Go to the greenhouse and perform normal management (temperature: 23-28°C during the day and 18-20°C at night). After culturing for 10 days, conduct an investigation and classify the pathogen-infected area. Use the disease index to calculate the control effect and obtain the EC90 value.
The test results show that 95% of the original drug triflufenacil has good activity against rice sheath blight, cucumber powdery mildew, and soybean rust.
【Preparation】 [1]
Using ethyl difluoroacetoacetate and triethyl orthoformate as starting materials, triethyl acetylacetate is synthesized through 6 steps of condensation, cyclization, hydrolysis, acid chlorination, and amidolysis. The synthesis route is shown in the figure. .
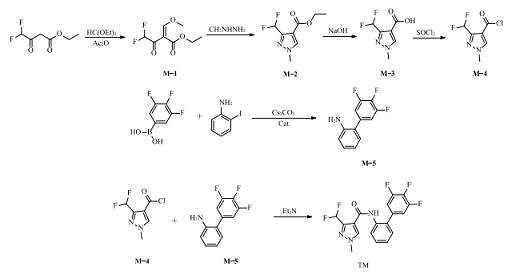
[Main reference materials]
[1] Wang Gang, Lu Liang, Liu Jiyong, et al. Synthesis and biological activity of triflufenac[J]. Modern Pesticides, 2017, 16(4): 12-14.
[2] Deng Jinbao. The U.S. EPA recommends registration of the new fungicide penflufen [J]. Pesticide Research and Application, 2012, 16(3): 35-35.
[3] Qin Enhao, Zhao Lili. Market research report on triflufenac and succinate dehydrogenase inhibitor fungicides (2) [J]. Agrichemical Market News, 2017 (1): 48 -51.



 微信扫一扫打赏
微信扫一扫打赏
