[Overview][1]
Biphenyldiphenol is an important organic intermediate that can be used as rubber anti-aging Agent and plastic antioxidant, it can be used in colorless vulcanized rubber products, rubber products for food packaging and medical latex products. It can also be used in sulfur chloride cold vulcanized products (such as medical gloves, condoms), etc. In terms of synthetic polymers, due to its excellent heat resistance, it can be used as a modified monomer for polyester, polyurethane, polycarbonate, polysulfone and epoxy resin to manufacture excellent engineering plastics and composite materials. etc. At present, its high-purity products are mainly used to synthesize liquid crystal polymers. Properties: White needle-like or flaky crystals. The melting point is 286℃ (274~275℃), and the relative density is 1.22. Easily soluble in ether, ethanol, ethyl acetate, acetone and sodium hydroxide, slightly soluble in benzene and methyl chloride, insoluble in gasoline, carbon tetrachloride and water.
[Preparation method][1]
1 Benzidine method
Benzidine is obtained by diazotization and hydrolysis. The reaction formula is as follows:
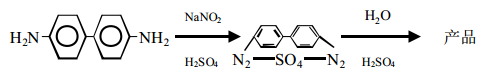
The process is: Dissolve benzidine in sulfuric acid medium to generate benzidine sulfate, and then diazotize it with sodium nitrite to obtain heavy benzidine. Nitrogen liquid is boiled and hydrolyzed in a dilute sulfuric acid medium to precipitate 4, 4,–biphenyl diphenol; then filtered, washed, dried, and refined by sublimation to obtain the finished product.
2 biphenyl sulfonation alkali fusion method
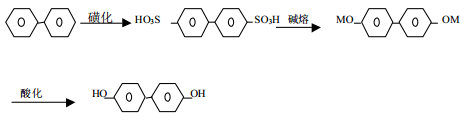
Biphenyl is obtained by sulfonation, alkali fusion and acidification. The reaction formula is as follows:
3 2,6-di-tert-butylphenol oxidative coupling and reductive dealkylation method
Uses 2,6-di-tert-butylphenol as raw material. The product is synthesized through a three-step process of oxidation, reduction and deisobutylene, and its product purity reaches electronic level (>99.5%). Reaction flow chart:
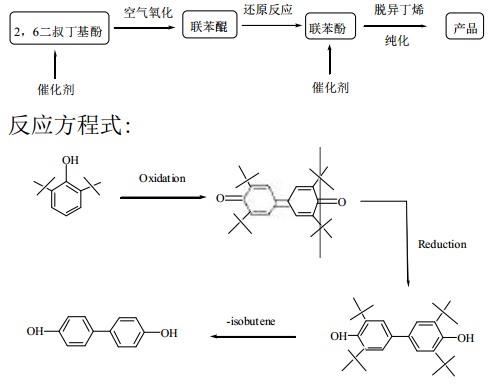
Preparation example: Add a mixture of 200g (0.968mol) of 2, 6-di-tert-butylphenol and 2g of 50% potassium hydroxide aqueous solution. In an autoclave with stirrer and thermostat. Heat to about 180°C and add oxygen until the internal pressure reaches 0.71Mpa. During this period, stirring was carried out at a speed of 460r/min. There is a slight exotherm in the initial stage, the temperature rises to about 200°C, and the oxygen pressure drops sharply to 0.53Mpa. During the 35min reaction time, oxygen was injected several times to bring the internal pressure to 0.71Mpa. After reacting for 35 minutes, the inside of the reactor was purged with nitrogen, and the product was analyzed by chromatography. Approximately 75% of the substituted phenol was converted into dimers. Then, the temperature of the reactant was raised to 300°C, and unreacted 2,6-di-tert-butylphenol was distilled off. As the dealkylation reaction proceeds, the temperature rises to 320°C and the by-product isobutylene is removed. After reacting for 4 hours, distill 4, 4, –biphenyldiol, to obtain a snow-white product with excellent quality.
Using 2, 6-di-tert-butylphenol as raw material, the product is synthesized through a three-step process of oxidation, reduction and deisobutylene, which avoids Use 4, 4, –Toxic compounds using diaminobiphenyl as raw materials have serious “three wastes”, poor product quality and many other fatal weaknesses. This method has simple process conditions, improved technical level, excellent product quality, no carcinogenic compounds, and high product quality. It is a new technology being developed abroad.
4 biphenyl halogenation hydrolysis method
Biphenyl is obtained through halogenation and hydrolysis. The reaction formula is as follows:
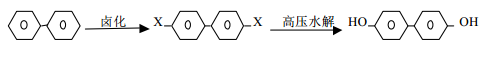
Biphenyl undergoes bromination reaction to generate 4, 4, – dibromobiphenyl, and sodium hydroxide under the action of catalyst at 300℃ Under the condition of temperature and pressure of 80kg/cm2, 4,4,-biphenyldiphenol is hydrolyzed.
[Application and Development Prospects]
The synthesis research of 4,4,-biphenyldiphenol in my country began in the 1980s and is mainly used as a plastic antioxidant. Dalian University of Technology, Xiamen University and other units have invested a lot of energy in researching the synthesis process of this product, mainly using the biphenyl disulfonation route, and have successively built several production lines. The main production companies include Suzhou Changtong Chemical Co., Ltd., Wujin Linchuan Chemical Industry Factory, Wuxian Longsheng Fine Chemical Factory, etc., with a total production capacity of 1,500t/a, and the actual output is below 1,000t/a. Foreign countries have begun research on the application of 4,4,-biphenyl diphenol in liquid crystal materials as early as the 1970s. In the 1990s, my country also invested a certain amount of energy in the research of related liquid crystal polymers. Now domestic Shijiazhuang, Yantai, Xi’an and other places All related liquid crystal polymer products have been put on the market. However, due to the limitations of synthesis technology, especially the low content of 4,4,-biphenyl diphenol, it has been unable to meet the needs of domestic and foreign markets.
Since 2001, several large foreign companies including Japan have proposed thousands of tons of high-purity liquid crystal material intermediates 4 to China, 4′- The market demand for biphenyldiphenol has triggered an upsurge in domestic development. Nanjing Normal University, Zhejiang University, Xishan Chemical Industry Research Institute of Jiangsu Province, Qingdao University of Science and Technology and other colleges and universities have successively invested a lot of manpower and material resources in high-purity research4. 4′ – Research and development of biphenyl diphenol, and has successively established production facilities in Yangcun, Beijing, Zhoushan, Zhejiang, Xinxiang, Henan, Zibo, Shandong, Nanjing, Jiangsu, Wuxi and other places. However, due to various reasons such as technology, production, and environmental protection, the current domestic production capacity of high-purity 4,4’-biphenyldiphenol has been very low, and demand exceeds supply.
4, 4, – Diphenol is a fine chemical product with promising market prospects. The current market situation analysis at home and abroad: this product The application in the rubber antioxidant industry no longer dominates. Synthetic liquid crystal polymer (LCP), which is mainly used in liquid crystal polymers (LCP), is the fastest growing special engineering plastic with high performance and new structure in recent years. It is a kind of High-performance materials with extremely superior comprehensive properties. Since 1995, the global demand for LCP has exceeded 10,000 tons. The United States, Japan, and Western Europe are important global LCP consumer markets. LCP has (1) good processability (2) self-reinforcement (3) excellent heat resistance (4) Small linear expansion coefficient (5) Excellent electrical properties (6) Excellent physical and chemical properties such as chemical resistance, so it is widely used in electronic parts, precision machinery parts, home appliance accessories, medical equipment, and automobile parts and chemical equipment parts and other fields.
LCP, which has the advantages of high heat resistance, high rigidity, thin-wall fluidity and low expansion, is a hot spot in research and development. A series of liquid crystal polymers developed using benzene as raw material have this characteristic. For example, the monomer methacrylic acid [5-(4′-methoxybiphenyl-4-oxy )pentyl] ester (M5MPP), 4-nitroazophenylmethyl-2-methacrylate ethylamine (MMEANB), monomers were polymerized and copolymerized to obtain nonlinear optics (NLO) The side chain liquid crystal polymer of the active group, due to the interaction between the electron-withdrawing and electron-donating groups between molecules, is beneficial to improving the thermal stability of the liquid crystal polymer, and the copolymer (M5MPP/MMEANB) has a wide liquid crystal phase temperature range.
Due to the unique properties of liquid crystal polymers synthesized from biphenyl diphenol, the market for this type of liquid crystal polymer has rapidly expanded. Development, since the advent of liquid crystal polymers using this product as raw material in 1972, has developed at a rapid pace, especially in recent years, with the advancement of computer and flat-panel TV technology and the development of various electronic equipment technologies, this The product provides huge market development space. According to conservative estimates: the current demand for high-purity biphenyl diphenol in the world’s liquid crystal material production industry has exceeded 7,000t.
my country has just started in the production of liquid crystal materials, but it is developing rapidly. In 1987, the research results of liquid crystals from the Department of Chemistry of Tsinghua University were published in Shijiazhuang Mass production began and was supplied to LCD factories. This company, now named Shijiazhuang Yongsheng Huaqing, is still the LCD material factory with the largest variety and largest output in China. Tsinghua Yawang Liquid Crystal Materials Co., Ltd. can also mass-produce TN liquid crystals and mid- to low-end STN liquid crystal materials. The market share of mid-to-low-end liquid crystal materials of Shijiazhuang Yongsheng Huaqing and Tsinghua Yawang has accounted for more than 70% of the market in mainland China, and low-end TN accounts for more than 80%. They also provide monomers to other liquid crystal material companies in Japan and Germany.
In addition to these two companies, Xi’an Ruilian Company is also engaged in the research and development and production of liquid crystal materials, but it mainly focuses on liquid crystal monomers. Equipped with partial hybrid LCD production. In addition, many companies in Jiangsu and Yantai are engaged in the development and production of monomers and intermediates of liquid crystal materials. At present, Shijiazhuang, Yantai, Xi’an and other places in China have begun to use 4,4,-biphenyl diphenol as raw material for production. The annual demand for liquid crystal polymer is around 100t, so the current market for this product is mainly exported.
[References]
[1] Yang Shanyue, Zhao Meifa. Important liquid crystal intermediate—biphenyl diphenol[J]. Chemical Technology Market, 2005(12) :33-35.
The market for this product mainly lies in export.
[References]
[1] Yang Shanyue, Zhao Meifa. Important liquid crystal intermediate—biphenyl diphenol[J]. Chemical Technology Market, 2005(12) :33-35.



 微信扫一扫打赏
微信扫一扫打赏
