[Overview]
Benzoic acid Also known as benzoic acid, it is the simplest aromatic acid in which the carboxyl group is directly connected to the carbon atom of the benzene ring, as free acid, vinegar or its derivatives It exists widely in nature, for example, it exists in the form of free acid and benzyl ester in benzoin gum, in the form of methyl ester or benzyl ester in some flavors, and in the leaves and stems of some plants as free acid. It exists in the urine of herbivores as hippuric acid, a derivative of benzoic acid. Benzoic acid is an important chemical raw material and product and is widely used in food, medicine, fine chemicals and other industries.
[Physical and chemical properties]
Benzoic acid, also known as benzoic acid, is the simplest aromatic acid with the carboxyl group directly connected to the carbon atom of the benzene ring. It is weakly acidic with an ionization constant of 4.20. It is more acidic than medium and long-chain fatty acids and has the properties of formaldehyde or benzene. Pungent smell. It appears as needle-like or scale-like crystals and is mainly found in different rosin esters, fruits and berries, especially Vaccinium species. It is also found in milk, milk products, animal tissues and secretory glands. Benzoic acid is slightly soluble in water and easily soluble in organic solvents such as ether and ethanol. It has a boiling point of 249°C, a melting point of 122.13°C, and a relative density of 1.2659 (15/4°C). It sublimates rapidly at 100°C, and its vapor is highly irritating and can easily cause coughing after inhalation.
Benzoic acid is not easily oxidized, and electrophilic substitution reactions can occur on its benzene ring, mainly to obtain meta-substitution products. The resulting derivatives are also widely used in the food and chemical industries for preservation and quality preservation. Benzoic acid is stable under normal temperature and pressure and is not prone to reaction. Its chemical properties mainly depend on the activity of functional groups, namely benzene ring and carboxyl group. Reactions on the carboxyl group include reaction with a base to form a salt, reaction with an alcohol to form the corresponding ester, substitution of the hydroxyl group by chlorine to form benzoyl chloride, substitution by an oxygen group to form benzamide, etc. The hydrogen atoms on the benzene ring in benzoic acid can be substituted by various atoms or groups, but since the carboxyl group on the benzene ring is a meta-positioned electron-withdrawing group that can purify the benzene ring, the sulfonation, nitration and Substitution reactions such as chlorination are more difficult than benzene. Hydrogenation reaction can also be carried out on the benzene ring. Benzoic acid is hydrogenated in the presence of catalyst to generate hexahydrobenzoic acid, which is an intermediate in the production of caprolactam. Benzoic acid can also undergo decarboxylation at high temperatures to produce benzene and carbon dioxide.
【Mechanism of action】
The antiseptic mechanism of benzoic acid is that it is highly lipophilic and can easily penetrate the cell membrane into the cell body, thereby interfering with the permeability of microbial cells such as bacteria and molds and inhibiting the absorption of amino acids by the cell membrane. The benzoic acid molecules entering the cells can ionize and acidify the alkali stored in the cells, inhibit the activity of the cell’s respiratory enzyme system, and prevent the condensation reaction of acetyl-CoA, thus playing a food preservative role. Benzoates can be converted into the effective form of benzoic acid in acidic foods, and its antibacterial effects are the same as benzoic acid.
[Pharmacokinetics]
Benzoic acidAfter entering the body, it is absorbed into the liver through the small intestine and excreted from the urine in four ways: one is to combine with glycine under the catalysis of enzymes It is synthesized into hippuric acid and excreted in the urine; the other is synthesized with glucuronic acid to form benzoyl glucuronic acid and excreted from the kidneys; it can also be excreted in the form of benzoic acid and ornithine. Some scholars also believe that benzoic acid in urine is produced by the degradation of benzoyl glucuronic acid. After humans ingest benzoic acid, it is almost completely excreted in the urine as hippuric acid within 24 hours, with no residue in the body. After large white pigs ingest benzoic acid, 85.93% of the benzoic acid is excreted in the form of hippuric acid and 15.70% is excreted in the form of benzoic acid within 24 hours, with basically no residue in the body. The most important metabolic pathway for most organic acids is to be oxidized into carbon dioxide in the body and excreted through the lungs. Since benzoic acid is mostly excreted in the urine in the form of hippuric acid, it is rarely enriched in the body. Therefore, adding benzoic acid to pig feed will not increase the acid load and disturb the body’s acid-base balance.
[Preparation method]
1. Toluene liquid phase air oxidation method. The commonly used catalyst is soluble cobalt salt or manganese salt, with acetic acid as the solvent.
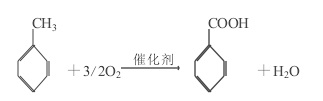
Figure 1 is the reaction equation of toluene liquid phase air oxidation method
The reaction mechanism is a free radical reaction, the reaction temperature is about 165°C, the pressure is 0.6~0.8MPa, and the reaction is an exothermic reaction. The main by-products are benzaldehyde, benzyl alcohol, o-methylbiphenyl, biphenyl, p-methylbiphenyl and esters. All by-products can be recycled and utilized, especially benzaldehyde and benzyl alcohol, whose unit price is often 4 to 5 times that of benzoic acid, which can greatly increase the output value and profit of the device.
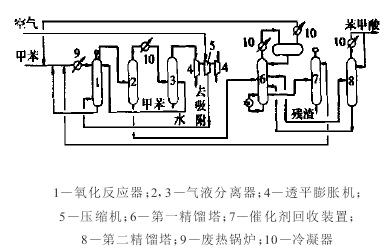
Figure 2 is a schematic flow chart of the production of benzoic acid by liquid-phase air oxidation of toluene
2. Toluene chlorination hydrolysis method: Toluene is photochlorinated at 100-150°C to obtain trichlorobenzylbenzene, which reacts with water in the presence of ZnCl2 (or lime milk and iron powder) to obtain benzoic acid. Based on trichlorobenzylbenzene, the benzoic acid yield is 74% to 80%. The reaction formula is:
<img alt="Toluene chloride hydrolysis method�The largest amount of preservatives used in my country is in the pharmaceutical industry. Benzoic acid can be used to produce diatrizoic acid, aceiodobenzoic acid, m-nitrobenzoic acid, 3,5-dinitrobenzoic acid, and 3,5-diaminobenzoic acid. etc.; in the dye industry, it is used to produce mordants such as 1,5-dihydroxyanthraquinone, benzoyl gas, etc.; in addition, benzoic acid is also used to manufacture plasticizers and is used as raw material for the production of phenol and caprolactam. It is also used for fiber processing.
[Main reference materials]
[1] Gao Zengbing, Yu Bing, Diao Hui, Yan Honglin, Chen Daiwen. Application of benzoic acid in pig and poultry feed [J]. Journal of Animal Nutrition, 2014, 26(05): 1127-1133.
[2]Wu Xingan, Chen Shuva. Synthesis and purification of benzoic acid[J]. Modern Chemicals, 2000(08):10-14.
[3]Xie Chicheng. Simulation and energy-saving scheme design of benzoic acid separation process[D]. Zhejiang University, 2012.
[4]Wu Jun. Research on new purification process of benzoic acid[D]. Wuhan University of Technology, 2014.



 微信扫一扫打赏
微信扫一扫打赏
