Background and overview[1]
As a new type of chemical cold light source, bisoxalate is a type of bisoxalate. It is a high-brightness, high-efficiency chemiluminescent substance that is environmentally friendly, non-charged, and safe. There are two methods for preparing bisoxalate esters in the existing technology. One process uses equivalent amounts of metallic sodium or sodium hydride, resulting in high costs; a large amount of sodium chloride solid is generated after the reaction. When water is added to wash the sodium chloride solid, The product bisoxalate decomposes when exposed to water, resulting in low yield and poor product quality. Another process will produce a large amount of triethylamine hydrochloride or n-butylamine hydrochloride solid waste, which needs to be filtered out when separating the product. Triethylamine hydrochloride or n-butylamine hydrochloride is particularly easy to absorb moisture. It makes filtration and transfer very difficult. When recovering triethylamine or n-butylamine from triethylamine hydrochloride or n-butylamine hydrochloride, the water contained in it has a great influence on the reaction, and the yield is lower, that is, it cannot be applied. Easy to implement; using a large amount of triethylamine or n-butylamine as acid acid agent not only results in high cost, but also makes it difficult to remove the water in triethylamine or n-butylamine, and the product bisoxalate is unstable , decomposes when exposed to water and alkali, so the reaction yield is very low. The low yield causes the product to be refined, and the refining is very troublesome; the reaction time is also long. .
Structure
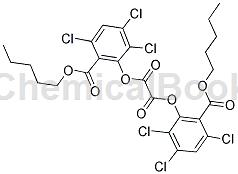
Preparation[1]
The preparation method of bisoxalate includes the following steps:
S1: Inflate. The reaction kettle 13 and the charging kettle 11 are filled with nitrogen for protection to avoid oxidative deterioration of the raw materials in contact with air.
S2: Adding materials. Add 1200L toluene, 0.7kg catalyst and 700kg amyl trichlorosalicylate into the reaction kettle 13, and add 140kg oxalyl chloride into the addition kettle.
S3: Pressurized. Continue to fill the reaction kettle 13 and the charging kettle 11 with nitrogen and pressurize it to 1kgf/cm2 to prevent water vapor, oxygen, etc. in the air from entering the reaction kettle 13 and interfering with production.
S4: Mixing. The reaction kettle 13 was heated to 103°C, and the raw materials were stirred for 0.5 hours, so that amyl trichlorosalicylate was dissolved in toluene and the catalyst was suspended in toluene. The reaction rate to form bisoxalate is faster at this temperature.
S5: Reaction. Start the diaphragm pump 12, and add the oxalyl chloride in the charging kettle 11 into the reaction kettle 13 at a uniform speed within 0.5 hours. Reaction begins in the reaction kettle 13 to generate bisoxalate.
S6: Insulation. After the addition of oxalyl chloride is completed, continue to heat to between 103°C and 106°C, keep warm, and continue the reflux reaction for 0.5 hours.
S7: Discharging. After the reaction is completed, amyl trichlorosalicylate is basically converted into bis(2,4,5-n-pentyl trichlorosalicylate) oxalate. The reaction kettle 13 is cooled to room temperature, the filter press 14 hydraulically transfers the reaction in the reaction kettle 13 to the distillation kettle 15 , and the catalyst is filtered out and recovered to the reaction kettle 13 .
S8: Drying under reduced pressure. The vacuum pump 16 is started to extract the gas in the distillation kettle 15 so that the vacuum state in the distillation kettle 15 is maintained at a pressure of minus 0.09Mpa. As the air pressure decreases, the boiling point of toluene also decreases, and the toluene is quickly evaporated to dryness to obtain the pure bis(2,4,5-trichlorosalicylic acid n-amyl ester) oxalate product. The finished product content is 97% and the yield is 97%.
Apply[2-4]
As a new type of chemical cold light source, bisoxalate has the following application examples:
1) Prepare a color-changing fluorescent stick, including a sealed and bendable plastic tube. The plastic tube has a glass tube inside. The plastic tube is filled with an activator solution. The glass tube is filled with a bis-oxalate solution and bis-oxalic acid. The ester solution is composed of the following mass fraction components: 20-30 parts of dibutyl phthalate, 15-25 parts of bis(2,4-dinitro-6-alkoxycarbonylphenyl)oxalate, 5, 0.2-0.7 parts of 12-(phenylethynyl)-butanol, 0.1-0.3 parts of 5,6,11,12-tetraphenylnaphthocene, 1-2 parts of 9,10-bis(styryl)anthracene; The activator solution is composed of the following mass fraction components: 40-45 parts of triethyl citrate, 5-10 parts of hydrogen peroxide, 0.1-0.6 parts of sodium salicylate, and 3-8 parts of water. The fluorescent rod of the present invention can change color during the light-emitting process, the change is obvious, and the total light-emitting time is long.
2) Prepare a luminescent composition. The luminescent composition includes waxy material, bisoxalate and fluorescent agent. The waxy material accounts for 15% to 98.69% of the total weight of the luminescent composition, preferably 25% to 85%; the fluorescent agent accounts for 0.01% to 5% of the total weight of the luminescent composition. Preferably 0.1%~1%. The percentage of the bisoxalate ester in the total weight of the luminescent composition is 1% to 60%, preferably 5% to 35%. The luminescent composition provided by the invention adopts a wax-based bisoxalate system, which overcomes the shortcomings of current luminescent pens, is a good substitute for existing chemical luminescent pens, and has important application value.
3) Prepare a breathable finishing agent for embroidery, weigh water, dinitrophenol, active agent, dibasic acid, carboxymethyl cellulose, polyethylene glycol distearate, magnesium sulfate hexahydrate, Ethyl cellulose, sodium alginate, bisoxalate and ethylene glycol; simple preparation method, good breathability, good anti-mildew effect, tensile strength 3-5MPa, excellent moisture resistance; simple process, high gloss, Adhesion level 1, good corrosion resistance, good abrasion resistance, high adhesive strength; anti-shrinkage, easy to use, washability 65-85 times, mite avoidance rate 92.5-96.5%, flexibility 1mm, good gloss; cost Low cost, extended service life of embroidery, fast drying, good flame retardant performance, good antibacterial performance, good for large intestineThe bacteriostatic rate is 98.8-99.2%, and it can be widely produced and continuously replace existing materials.
Main reference materials
[1] CN201510468478.1 Bisoxalate preparation device and preparation method thereof
[2] CN201710695643.6 A color-changing fluorescent stick
[3] CN201110279405.X luminescent composition and its preparation method and application
[4] CN201711238694.2 Preparation method of breathable finishing agent for embroidery



 微信扫一扫打赏
微信扫一扫打赏
