
Structural formula
| Business number | 02EL |
|---|---|
| Molecular formula | C9H12 |
| Molecular weight | 120 |
| label |
cumene, Cumene, (1-Methylethyl)benzene, Aromatic hydrocarbons |
Numbering system
CAS number:98-82-8
MDL number:MFCD00008881
EINECS number:202-704-5
RTECS number:GR8575000
BRN number:1236613
PubChem number:24862376
Physical property data
1. Properties: colorless liquid with special aromatic odor. [1]
2. Melting point (℃): -96.0[2]
3. Boiling point (℃): 152.4[3]
4. Relative density (water = 1): 0.86[4]
5. Relative vapor Density (air=1): 4.1[5]
6. Saturated vapor pressure (kPa): 2.48 (50℃)[6]
7. Heat of combustion (kJ/mol): -4951.8[7]
8. Critical temperature (℃): 362.7[8]
9. Critical pressure (MPa): 3.21[9]
10. Octanol/water partition coefficient: 3.66 [10]
11. Flash point (℃): 31[11]
12. Ignition temperature (℃): 424 [12]
13. Explosion upper limit (%): 6.5[13]
14. Explosion lower limit (%) : 0.9[14]
15. Solubility: Insoluble in water, soluble in most organic solvents such as ethanol, ether, benzene, carbon tetrachloride, acetone, etc. [15]
16. Eccentricity factor: 0.338
17. van der Waals area (cm2·mol-1): 1.014×1010
18. van der Waals volume (cm3·mol-1 ): 79.960
19. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5260.59
20. Gas phase Standard claimed heat (enthalpy) (kJ·mol-1): 4.02
21. Gas phase standard entropy (J·mol-1·K-1): 386.11
22. Gas phase standard free energy of formation (kJ·mol-1): 139.1
23. Gas phase standard hot melt (J·mol-1·K-1): 159.69
24. Liquid phase standard combustion heat (enthalpy) (kJ ·mol-1): -5215.44
25. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -41.13
26. Liquid phase standard entropy (J·mol-1·K-1): 277.57
27. Liquid phase Standard free energy of formation (kJ·mol-1): 125.09
28. Liquid phase standard hot melt (J·mol-1·K-1):215.40
Toxicological data
1. Acute toxicity[16]
LD50: 1400mg/kg (rat oral); 12300μl (10578mg)/kg ( Rabbit transdermal)
LC50: 15300mg/m3 (mouse inhalation, 2h)
2. Irritation[17 ]
Rabbit transdermal: 100mg (24h), moderate irritation.
Rabbit eye: 500mg (24h), mild irritation.
3. Subacute and chronic toxicity [18] Rats inhaled 2.5g/m3, 8 hours a day, 6 days a week for a total of 150 days, obvious congestion in the lungs, liver, and kidneys was seen.
4. Mutagenicity[19] Microbial mutagenicity: Salmonella typhimurium 100μg/dish (3h)
Ecological data
1. Ecotoxicity[20] LC50: 6.32mg/L (96h) (fathead minnow)
2. Biodegradability [21] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, degradation 26.1%~40% after 2 weeks.
3. Non-biodegradability[22] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 2.5d (theoretical).
4. Bioaccumulation [23] BCF: 35.5 (goldfish, contact concentration 1mg/L)
Molecular structure data
1. Molar refractive index: 40.43
2. Molar volume (cm3/mol): 139.5
3. Isotonic specific volume (90.2K ): 322.3
4. Surface tension (dyne/cm): 28.4
5. Dielectric constant: 2.32
6. Dipole moment (10-24cm3):
7. Polarizability: 16.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 68.1
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Oxidize with dilute nitric acid or chromic acid to generate benzoic acid. Nitration reaction occurs with fuming nitric acid in the presence of acetic anhydride or acetic acid to produce 2,4-dinitrocumene. When reacting with concentrated sulfuric acid, sulfonation reaction mainly occurs at the para position. Under ultraviolet irradiation, oxygen is introduced at 85°C or at 90-130°C and 0.1-1MPa, oxygen is introduced for oxidation to generate cumene hydroperoxide. Under the catalysis of sulfuric acid or acidic ion exchange resin, cumene hydroperoxide decomposes into phenol and acetone. Cumene is decomposed into benzene and propylene under the catalysis of aluminum silicate at 400 to 500°C.
2. Stability[24] Stable
3. Incompatible substances[25] Strong oxidants, acids, halogens, etc.
4. Polymerization hazards[26] No polymerization
Storage method
Storage Precautions[27] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the alkylation reaction of benzene and propylene. Aluminum trichloride is usually used as the catalyst and hydrogen chloride is used as the accelerator. The reaction is carried out at normal pressure and around 95°C. In addition to cumene, polyalkyl by-products such as dicumyl and tricumyl are also produced. In order to reduce side reactions, excess benzene can be used. The molar ratio of benzene to propylene is about 3; the content of aluminum trichloride in the reaction solution is 3%-8%; the reaction is carried out at a low propylene concentration. In order to improve the production capacity of the reactor and reduce the loss of benzene in the tail gas, the reaction pressure can be increased to 0.5-0.6MPa. The alkylated liquid obtained from the reaction is cooled and precipitated, and the separated solid (aluminum trichloride and polycumyl complex) is recycled. The alkylated liquid is hydrolyzed, neutralized and distilled to obtain cumene. Another gas phase process is to pass gaseous benzene and propylene through a phosphoric acid catalytic bed loaded on alumina or aluminum silicate to perform catalytic alkylation. The reaction pressure is 1.5-40MPa and the temperature is about 250°C. At present, the industrial production of cumene is mainly based on the above-mentioned liquid phase method.
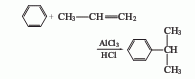
2. The preparation method is to Benzene and propylene enter the alkylation tower together in the presence of the catalyst aluminum trichloride. Benzene contains a small amount of hydrogen chloride as a cocatalyst. The reaction temperature is 95 to 100°C. The reaction product contains 30% to 35% of cumene and polycumene. 10%~15%. The reaction liquid is cooled, separated by sedimentation, and the complex of aluminum trichloride in polycumene is separated. The ethylbenzene is removed, and the crude cumene is distilled to obtain the finished product.
Purpose
1. Mainly used in the production of phenol, acetone, a-methylstyrene, cumene hydroperoxide, etc., and can also be used as additives to increase the octane number of fuel oil, synthetic fragrances and polymerization initiators. raw material.
2. It is an intermediate in the production of the herbicide isopropuron (N-4-isopropylphenyl-N’,N’-dimethylurea) and an important intermediate in the production of phenol.
3. Used in organic synthesis and as solvent. [28]



 微信扫一扫打赏
微信扫一扫打赏
