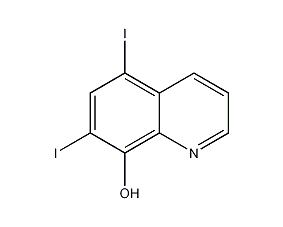
Structural formula
| Business number | 01U9 |
|---|---|
| Molecular formula | C9H5I2NO |
| Molecular weight | 396.95 |
| label |
5,7-diiodo-8-hydroxyquinoline, Dihydroxyquine, Diiodoquine, 5,7-Diiodoquinolin-8-ol, 5,7-Diiodo-8-quinolinol |
Numbering system
CAS number:83-73-8
MDL number:MFCD00006789
EINECS number:201-497-9
RTECS number:VC5775000
BRN number:None
PubChem number:24893382
Physical property data
1. Characteristics: light yellow to yellow-brown fine crystalline powder.
2. Density (g/mL ,25/4℃):Undetermined
3. Relative vapor density (g /mL,AIR= 1): Undetermined
4. Melting point (ºC): about214℃(decomposition)
5. Boiling point (ºC,Normal pressure): Undetermined
6. Boiling point (ºC,5.2 kPa): Undetermined
7. Refractive Index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific optical rotation (º ): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
Cat caliberLDLO:300mg/kg;
Pig caliberLDLO:50mg/kg;
2. Teratogenicity
E. coli: 260nmol/plate;
Mouse: 80 mg/kg;
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 69.88
2. Molar volume (m3/mol):159.3
3. isotonic specific volume (90.2K):471.4
4. Surface Tension (dyne/cm):76.6
5. Polarizability(10-24cm3):27.70
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 33.1
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 191
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed in Store in a cool and dry place.
Synthesis method
By8-Hydroxyquinoline is obtained by iodination.
Purpose
Used as anti-amidamide Pakistani medicine, antiseptics, metal chelating agents, cation analysis reagents. Diiodoquinoline, quiniodoform, and clioiodoquinoline are all halogenated8-Hydroxyquinoline intestinal anti-amoebic drugs are effective against amoebic dysentery and have no effect on extraintestinal amebic protozoa. In recent years, it has been reported abroad that this type of drug can cause subacute myelooptic neuropathy, so it has been banned in Japan and the United States. However, this disease is less common with iodoquinoline than with clioquinoline.
fareast-font-family: Arial”>4. Surface Tension (dyne/cm):76.6
5. Polarizability(10-24cm3):27.70
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 33.1
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 191
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None yet
Storage method
This product should be sealed in Store in a cool and dry place.
Synthesis method
By8-Hydroxyquinoline is obtained by iodination.
Purpose
Used as anti-amidamide Pakistani medicine, antiseptics, metal chelating agents, cation analysis reagents. Diiodoquinoline, quiniodoform, and clioiodoquinoline are all halogenated8-Hydroxyquinoline intestinal anti-amoebic drugs are effective against amoebic dysentery and have no effect on extraintestinal amebic protozoa. In recent years, it has been reported abroad that this type of drug can cause subacute myelooptic neuropathy, so it has been banned in Japan and the United States. However, this disease is less common with iodoquinoline than with clioquinoline.
FAMILY: Arial; mso-font-kerning: 0pt”>



 微信扫一扫打赏
微信扫一扫打赏
