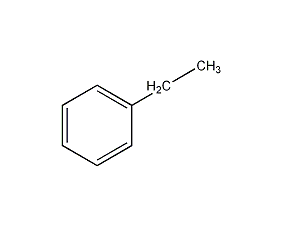
Structural formula
| Business number | 02HU |
|---|---|
| Molecular formula | C8H10 |
| Molecular weight | 106.17 |
| label |
Ethylbenzene, phenylethane, Phenethane, Aethylbenzol, Phenylethane, Nitro spray paint thinner, organic synthesis solvents, cellulose ether solvent, For synthesis of synmycin, Aromatic hydrocarbons |
Numbering system
CAS number:100-41-4
MDL number:MFCD00011647
EINECS number:202-849-4
RTECS number:DA0700000
BRN number:1901871
PubChem number:24889462
Physical property data
1. Properties: Colorless and transparent liquid with aromatic odor. [1]
2. Melting point (℃): -94.9[2]
3. Boiling point (℃): 136.2[3]
4. Relative density (water=1): 0.87 (20℃)[4]
5. Relative vapor density (air = 1): 3.66[5]
6. Saturated vapor pressure (kPa): 0.9 (20℃)[6]
7. Heat of combustion (kJ/mol): -4390.1[7]
8. Critical temperature (℃): 344.1[8]
9. Critical pressure (MPa): 3.60[9]
10. Octanol/water partition coefficient: 3.15[10]
11. Flash point (℃): 12.8 (CC) [11]
12. Introduction Ignition temperature (℃): 432[12]
13. Explosion limit (%): 6.7[13]
14. Lower explosion limit (%): 1.0[14]
15. Solubility: Insoluble in water, miscible in most organic solvents such as ethanol, ether, and benzene. [15]
16. Viscosity (mPa·s, 25ºC): 0.6354
17. Heat of evaporation (KJ/mol, 25ºC): 42.278
18. Heat of fusion (KJ/mol, -94.975ºC, 101.3kPa): 9.190
19. Heat of formation (KJ/mol, 25ºC, gas): 29.810
20. Heat of formation (KJ/mol, 25ºC, liquid): -12.464
21. Heat of combustion (KJ/mol, 25ºC, gas): 4610.21 (H2O liquid): 4389.98
22. Heat of combustion (KJ/mol, 25ºC, liquid): 4567.92: 4347.69 (CO2 gas)
23. Specific heat capacity (KJ/(kg·K), 298.16K): 1.21
24. Solubility (%, 25ºC, water): 0.0152
25. Volume expansion coefficient (K-1, 0~40ºC): 0.00056
p>
26. Critical density (g·cm-3): 0.284
27. Critical volume (cm3·mol-1): 374
28.. Critical compression factor: 0.2643
29. Eccentricity factor: 0.304
30. Solubility parameter (J·cm-3)0.5:18.043
31. van der Waals area (cm2·mol-1): 8.800×109
32. van der Waals volume (cm3·mol– 1): 69.740
33. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4607.13
34. Gas phase standard claims heat (enthalpy) (kJ·mol-1): 29.92
h2 id=”hc”>Synthesis method
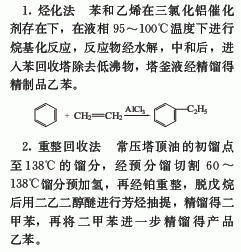
4. The liquid phase alkylation method is usually At normal pressure and 85-90°C, aluminum trichloride is used as a catalyst to react ethylene and benzene to produce ethylbenzene. A side reaction is that ethylbenzene is further alkylated with ethylene to form polyethylbenzene. Industrially, the benzene conversion rate is limited to 52-55%, and a high benzene-ethylene molar ratio (generally around 2) is used to prevent the formation of more diethylbenzene and polyethylbenzene. The average yield of ethylbenzene is 94-96%.
5. The gas-phase alkylation method initially involves gas-phase alkylation of ethylene and excess benzene under the action of phosphoric acid-diatomite on alumina-silica gel catalyst at 300°C and 4-6 MPa. Ethylbenzene is produced through sylation reaction. Since the catalyst used cannot dealkylate polyethylbenzene, polyethylbenzene cannot be treated. Although the production of polyethylbenzene is reduced by increasing the benzene batching ratio, it increases the distillation cost of recycled benzene.
6. Separation of C8 aromatic hydrocarbons to produce ethylbenzene and aromatic hydrocarbons produced by catalytic reforming. After benzene and toluene are separated and removed, the boiling points of the components of the mixed xylene fraction are very close. It takes 300-400 trays to separate ethylbenzene by distillation, and the reflux ratio is 75. In addition, adsorption separation and chromatography can also be used to resolve ethylbenzene. Since the separation of ethylbenzene from C8 aromatics is no longer economically competitive with benzene alkylation of ethylbenzene, and a new generation of precious metal isomerization catalysts can effectively convert ethylbenzene into xylene, the importance of ethylbenzene separation has greatly increased. decline.
Purpose
1. The main use is to produce styrene through dehydrogenation. Others are used as intermediates of synmycin in medicine. Also used as diluent for nitrocellulose spray paint and solvent for organic synthesis. It becomes a good solvent for cellulose ethers when mixed with ethanol and ethyl acetate.
2. Used in organic synthesis and as solvent. [31]



 微信扫一扫打赏
微信扫一扫打赏
