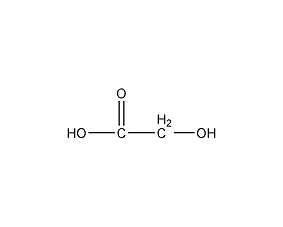
Structural formula
| Business number | 01PD |
|---|---|
| Molecular formula | C2H4O3 |
| Molecular weight | 76.05 |
| label |
glycolic acid, glycolic acid, a-glycolic acid, Hydroxyacetic acid, Ghycolic acid, Hydroxyethanoic acid, a-Hydroxyacetic acid, Multifunctional solvents, detergent, acidic solvent |
Numbering system
CAS number:79-14-1
MDL number:MFCD00004312
EINECS number:201-180-5
RTECS number:MC5250000
BRN number:1209322
PubChem number:24847624
Physical property data
1. Properties: The pure product is colorless, easily deliquescent crystal, flammable.
2. Density (g/mL, 25/4℃): 1.416
3. Melting point (ºC): 75~80
4. Boiling point ( ºC, normal pressure): 112
5. Flash point (ºC): 300
6. Solubility: soluble in water, methanol, ethanol, acetone, acetic acid and ether. Virtually insoluble in hydrocarbon solvents.
7. Crystalline phase standard combustion heat (enthalpy) (kJ·mol-1): -695.0
8. Crystalline phase standard claim heat ( Enthalpy) (kJ·mol-1): -663.6
Toxicological data
1. Acute toxicity: rat caliber LD50: 1950mg/kg; cat intravenous LD50: 1mg/kg; Toxicity: Rabbit eye test: 2 mgREACTION SEVERITY
3. Other multiple dose toxicity data: rat caliber TCLO: 2480mg/kg/35W-C; rat inhalation LDLO: 2 gm/m3
4. It is toxic if taken orally. It is corrosive and can cause burns. Slightly irritating to skin and mucous membranes.
Ecological data
This product is irritating to the eyes, skin, mucous membranes and upper respiratory tract. Very severe burning effect on skin and eyes. Pure glycolic acid is slightly toxic when taken internally because it is highly acidic. It is best not to come into contact with the skin. Wear acid-proof clothing and protective goggles during operation.
Molecular structure data
1. Molar refractive index: 14.412, Molar volume (cm3/mol): 53.6
3, Isotonic specific volume (90.2K): 150.2
4, Surface tension ( dyne/cm): 61.3
5. Polarizability (10-24cm3): 5.71
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 57.5
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 40.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Colorless crystals, easy to deliquesce.
2.The pure product has low toxicity. However, because this product is a strong acid and irritating, severe swelling and pain may occur when it comes in contact with the skin. The oral LD50 in rats is 1950 mg/kg. On-site operators must wear protective equipment, production equipment must be strictly sealed, and the work site must have good ventilation equipment.
3. Exist in flue-cured tobacco leaves, burley tobacco leaves and smoke.
Storage method
1. This product should be sealed and stored in a cool, dark place.
2. Pack in glass bottles or lined iron drums. If the quantity is large, it can be transported in lined tank trucks. The tank trucks should be insulated and equipped with external heating pipes for unloading in cold weather. Packaging containers should have warning signs indicating no contact with skin, eyes and clothing.
Synthesis method
1. Chloroacetic acid method Chloroacetic acid is hydrolyzed under alkaline conditions to obtain a crude product, which is then esterified with methanol to obtain methyl glycolate, which is then distilled and then hydrolyzed to obtain the finished product.
![]()
2. High temperature and high pressure method consists of formaldehyde Produced by the reaction of carbon dioxide and water.
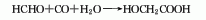
3.Formaldehyde and hydrocyanic acid react at normal pressure of 0 to 50°C under weakly alkaline or weakly acidic conditions. The resulting cyanomethanol is then hydrolyzed under acidic conditions, and the reaction temperature is controlled to be greater than 90 ℃, after the reaction is completed, use extraction and crystallization methods for purification, and the finished product can be obtained.
Process reaction formula:
![]()
4. The sodium cyanide method uses formaldehyde and sodium cyanide as raw materials and is produced by adding cyanide and acidic hydrolysis.
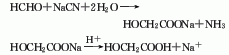
5.The finished product of glycolic acid is obtained by reacting chloroacetic acid with sodium hydride, esterifying with methanol under the action of sulfuric acid, then hydrolyzing, and recovering methanol through vacuum distillation. Or formaldehyde and carbon monoxide condense in the presence of acidic catalysts such as sulfuric acid or boron trifluoride to form glycolic acid.
6. Heat the chloroacetic acid solution to 85°C, add sodium hydroxide solution to react, and maintain the temperature at 95-98°C for 48 hours. Afterwards, remove sodium chloride and concentrate under reduced pressure to obtain the gum resin, and then heat and reflux until the gum resin is completely Dissolve in methanol, evaporate the remaining methanol, distill under reduced pressure to obtain ethyl glycolate, then hydrolyze, distill under reduced pressure until the content is acceptable.
The process reaction formula is:
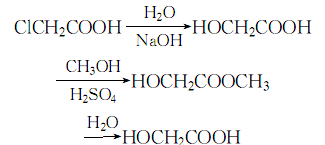
7. Tobacco: FC, 54; BU, 26.
Purpose
1. Raw materials for organic synthesis, which can be used to produce ethylene glycol. Glycolic acid is mainly used as a cleaning agent. A mixed acid composed of 2% glycolic acid and 1% formic acid is a high-efficiency and low-cost detergent, suitable for cleaning air conditioners; it can be used to prepare fiber dyes, detergents, welding agent ingredients, and varnishes Ingredients, copper etching agents, adhesives, petroleum demulsifiers and metal chelating agents, etc.; sodium salt and potassium salt of glycolic acid are used as electroplating solution additives. Other uses include electrolytic grinding, metal pickling, leather dyeing and tanning agents. It can also be used as a chemical analysis reagent.
2.Used as a cleaning agent, it can fully react with rust, calcium salts, magnesium salts, etc. in the equipment to achieve the purpose of descaling. It is easy to remove calcium carbonate scale and iron scale, and has good treatment effect. The corrosiveness to materials is very low, and organic acid iron will not precipitate during cleaning. Chemical cleaning with glycolic acid is less dangerous and easy to operate.
3.Used in organic synthesis and printing and dyeing industries
4.Used for sterilization of soap
5.Glycolic acid used for complexing of electroless nickel plating agent to improve the quality of plating, and can also be used as an additive for other electroplating or chemical plating.



 微信扫一扫打赏
微信扫一扫打赏
