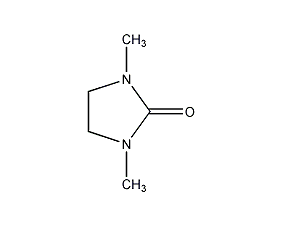
Structural formula
| Business number | 01RG |
|---|---|
| Molecular formula | C5H10N2O |
| Molecular weight | 114.15 |
| label |
1,3-dimethylimidazolinone; N,N′-dimethyl five-membered cyclic urea, 1,3-dimethyl imidazolidinone, N, N’-dimethyl five-membered ring urea, stripper |
Numbering system
CAS number:80-73-9
MDL number:MFCD00003188
EINECS number:201-304-8
RTECS number:NJ0660000
BRN number:108808
PubChem number:24865436
Physical property data
1. Physical property data
1. Properties: colorless, transparent, low-viscosity liquid.
2. Density (g/mL, 25/4℃): 1.044
3. Relative vapor density (g/mL, air=1): Uncertain
4. Melting point (ºC): 8.2
5. Boiling point (ºC, normal pressure): 225.0
6. Boiling point (ºC, 5.2kPa): Uncertain
p>
7. Refractive index: 1.471-1.473
8. Flash point (ºC): 107.0
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure ( kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/ V): Uncertain
18. Lower explosion limit (%, V/V): Uncertain
19. Solubility: It is a highly polar aprotic solvent that not only dissolves organic The compound also dissolves many inorganic compounds and has activating properties for various reagents. It is not easily hydrolyzed in aqueous solution, stable in hot alkaline solution or acidic solution, and stable to light and oxygen in the air under normal conditions.
Toxicological data
1. Acute toxicity: rat caliber LD50: 1190 uL/kg; mouse abdominal cavity LD50: 2840g/kg; rabbit skin LD50: 930 uL/kg.
2. Neurotoxicity: rabbit eye test: 100mg/24H.
3. Tests on rabbit skin show that it neither causes skin irritation nor allergic reactions.
Ecological data
None yet
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 30.51
2. Molar volume (cm3/mol): 107.3
3. Isotonic specific volume (90.2K): 258.4
4. Surface tension (dyne/cm): 33.6
5. Polarizability (10-24cm3): 12.09
Compute chemical data
1. Reference value for calculation of hydrophobic parameters (XlogP): -0.5
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 23.6
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 101
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Colorless, transparent, low-viscosity liquid, non-toxic.
It is a highly polar aprotic solvent that not only dissolves organic compounds but also many inorganic compounds, and has activation properties for various reagents. It is not easily hydrolyzed in aqueous solution, stable in hot alkaline solution or acidic solution, and stable to light and oxygen in the air under normal conditions.
Storage method
Store sealed in a cool, ventilated, dry place.
Synthesis method
First, urea and ethylenediamine undergo a ring-closing reaction in an ethylene glycol solution to generate 2-imidazolinone, and then 2-imidazolinone reacts with formaldehyde and formic acid to generate 1,3-DMI. 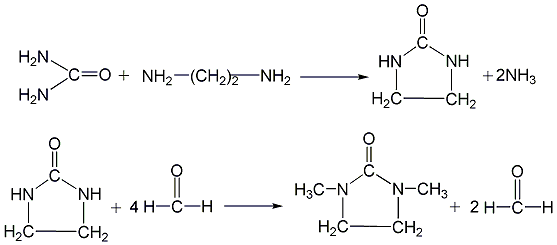
Purpose
This product is an aprotic polar solvent. It has excellent dissolving properties for many inorganic and organic compounds, including various resins. It is used as a reaction solvent for polymer synthesis, pesticides, and medicines. Its high dielectric constant and excellent Solvating properties can activate the reaction reagents during chemical reactions, speed up the reaction speed, and increase product yield. Due to its unique characteristics of strong polarity, high boiling point, high flash point, low melting point, and non-toxicity, it can effectively replace dimethylformamide (DMF) and the carcinogenic hexamethylphosphoryltriamide (HMPA). ). This product is also used as a dissolving agent for insoluble substances (dyes, pigments, polymers), surface cleaning agent, stripping agent, etc.
DMI has low toxicity. Experiments on rabbit skin have shown that it neither causes skin irritation nor allergic reactions; it has a high boiling point and flash point. After using it as a solvent, it can be easily distilled, filtered, and extracted. Recycling by other methods; compared with other aprotic polar solvents, DMI also has the advantages of being difficult to hydrolyze in aqueous solutions, stable in hot alkaline solutions or acidic solutions, and stable to light and oxygen in the air under normal conditions.



 微信扫一扫打赏
微信扫一扫打赏
