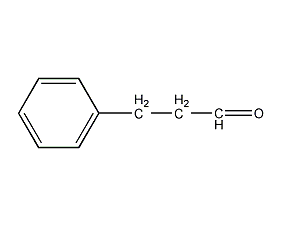
Structural formula
| Business number | 02Q8 |
|---|---|
| Molecular formula | C9H10O |
| Molecular weight | 134.18 |
| label |
benzyl acetaldehyde, phenylpropionaldehyde, Hydrogenated cinnamaldehyde, Hydrocinnamaldehyde, Hydrogenated cinnamaldehyde, Phenylpropanal, 3-Phenylpropan-1-al, Benzenepropanal, Hydrocinnamicaldehyde |
Numbering system
CAS number:104-53-0
MDL number:MFCD00007021
EINECS number:203-211-8
RTECS number:MW4890000
BRN number:1071910
PubChem number:24864504
Physical property data
1. Properties: colorless liquid with hyacinth-like aroma.
2. Density (g/mL, 25℃): 1.010~1.020
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 47
5. Boiling point (ºC, 99.2kpa): 221~224
6. Boiling point (ºC, kPa): Undetermined
7. Refractive index: 1.520~1.532
8. Flash point (ºC): 97
9. Specific rotation (º): Undetermined
7. p>
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg,ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol
Toxicological data
1. Skin/eye irritation: Standard Dresser test: human skin contact, 100%; 2. Acute toxicity: rat oral LD50: >5mg/kg; mouse intravenous LD50: 56mg/kg; rabbit skin contact LD50:>5mg/kg;
Ecological data
Usually not harmful to water.
Molecular structure data
1. Molar refractive index: 40.62
2. Molar volume (cm3/mol): 136.0
3. Isotonic specific volume (90.2K ): 332.5
4. Surface tension��dyne/cm): 35.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 16.10
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 2
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 92.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides, alkali and air.
2. Found in tobacco leaves.
3. Naturally found in essential oils such as cinnamon oil.
Storage method
1. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. It should be stored separately from oxidants, strong alkali and air, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. Prohibited 2. The use of mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials. 3. Storage temperature 4ºC
Synthesis method
1. Prepared by catalytic hydrogenation of cinnamic aldehyde.
2. Preparation method:

Into a reaction bottle equipped with a stirrer, thermometer, reflux condenser, and ventilation tube, add 2.88g (0.12mol) magnesium chips, 300mL anhydrous THP, and one grain of iodine. Pass dry nitrogen gas. Add dropwise a solution of 14.06 g (0.1 mol) of β-phenylethyl chloride (2) dissolved in 50 mL of THF. First add about 2 mL and warm to initiate the reaction. After the reaction is initiated, slowly add the rest of the solution dropwise, at a speed that allows the reaction solution to reflux slightly. After the addition was completed, the reaction was stirred at 23°C for 1 hour, and then refluxed for 8 hours. Cool to 0°C, slowly drop 13.56g (0.12mol) of N-formylpiperidine and dry hydrochloric acid to acidify to pH 2, separate the organic layer, and extract the aqueous layer three times with diethyl ether. Combine the organic layers, wash with water, 10% sodium bicarbonate, and saturated brine in sequence, dry over anhydrous magnesium sulfate, reflux the solvent and fractionate under reduced pressure to collect the fraction at about 87°C/133Pa to obtain 3-phenylpropionaldehyde (1 ) 8.8~10.2g, yield 66%~76%. [1]
Purpose
It is widely used in the preparation of various floral flavors, especially lilac, jasmine and rose flavors, and is also used in organic synthesis.



 微信扫一扫打赏
微信扫一扫打赏
