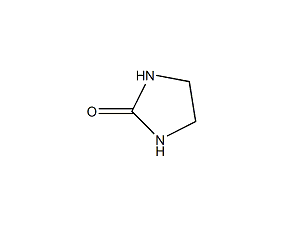
Structural formula
| Business number | 03D0 |
|---|---|
| Molecular formula | C3H6N2O |
| Molecular weight | 86.09 |
| label |
Cyclethylene urea, Cyclethylene urea, Ethylene urea, Cyclovinylidene urea, ethylene urea, 2-imidazolidinone, 1,3-ethylene-ure, 1,3-Ethyleneurea, 2-Oxoimidazolidine, 2-oxomidazolidine, Ethylenurea, Imidazolid-2-one, Imidazoliden-2-one, Imidazolidin-2-on, plasticizer, adhesive |
Numbering system
CAS number:120-93-4
MDL number:MFCD00005257
EINECS number:204-436-4
RTECS number:NJ0570000
BRN number:106252
PubChem number:24869298
Physical property data
Toxicological data
1. Acute toxicity: mouse peritoneal cavity LD50: 500mg/kg
2. Oncogenic toxicity: mouse subcutaneous TDLO: 1000mg/kg/
3. Reproduction Toxicity data: Rat (male) peritoneal cavity TDLO: 15mg/kg/1D
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 20.66
2. Molar volume (cm3/mol): 77.2
3. Isotonic specific volume (90.2K ): 182.1
4. Surface tension (dyne/cm): 30.8
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 8.19
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 2
6. Topological molecule polar surface area 41.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 63.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
It has irritating effects on glasses, respiratory tract, skin, etc. In case of contact�Eyes, rinse immediately with plenty of water and send to hospital for treatment.
Storage method
Keep away from fire and heat sources, seal and store in a cool, dry environment, away from moisture.
Synthesis method
1. Synthesis method using ethylenediamine and urea as raw materials
Feeding ratio (mass) ethylenediamine: urea: auxiliary = 1: (0.8~0.9): (0.3~0.5)
Add urea, ethylenediamine and additives in proportion to the reaction kettle, and slowly raise the temperature to boiling. After 0.5h, continue to heat up (the temperature rise rate must be controlled) to slowly recover the additives and excess ethylenediamine until the reaction temperature rises to 250°C, and the total time is controlled to about 5h. Incubate at 250°C for 2 hours, then cool and recrystallize to obtain white 2-imidazolidinone. Its melting point is 131~134℃, and the yield is greater than 95%.
2. Synthesis method using ethylenediamine and urea as raw materials
Feeding ratio (molar ratio) urea: ethylene glycol = 4:1
In Put urea and ethylene glycol into the reaction kettle respectively, start stirring, and slowly raise the temperature to 160~180°C within 6.5 hours; it takes another 3 hours to heat the reactant to 240°C. Then stir and react for 1 hour at 240°C to obtain a crude product. The crude product is subjected to simple vacuum distillation to obtain a fine product, with a yield of about 25% (based on ethylene glycol).
3. Synthesis method using carbon monoxide, sulfur and ethylenediamine as raw materials
Add 30g ethylenediamine, 16g sulfur and 30ml methanol into a 1.8L stainless steel autoclave. The carbon monoxide was introduced until it reached 0.9MPa and stopped. The temperature was raised to 100°C and reacted for 2 hours. Then cool, vent to remove hydrogen sulfide, rinse the residue with methanol, further cool, and filter to remove excess sulfur. The filtrate was concentrated to 75~100ml, cooled to precipitate crystals, then filtered and dried to obtain 22.4g of 2-imidazolidinone, melting point 131°C, yield 58%.
4. Synthesis method using phosgene and ethylenediamine as raw materials
In a 300ml four-necked flask equipped with a dropping funnel, reflux condenser, vent tube and mechanical stirring , add 100ml water, 12g ethylenediamine, and start stirring. Pour phosgene into the reaction kettle through the ventilation tube at a rate of 10g/h, while adding 20% sodium hydroxide aqueous solution dropwise, and the reaction temperature is controlled at 20°C. After 2 hours, the flow of phosgene was stopped, and stirring was continued for 1 hour at 20°C. After the reaction is completed, the reaction is distilled under reduced pressure, and the residue is recrystallized from chloroform. The yield can reach more than 80%. Melting point 131℃.
5. Other synthesis methods: reaction of ethylene carbonate and ammonia; reaction of COS and ethylenediamine; hydrolysis of ethylene diisocyanate; catalytic hydrogenation of diethyl ureidoacetate, etc.
Purpose
Used as a formaldehyde capture agent to remove residual formaldehyde from fabrics treated with 2D-resin, KB resin, urea-formaldehyde resin, melamine-formaldehyde resin, etc. This product is also used as an intermediate for fine chemicals in the manufacture of resins, plasticizers, spray paints, adhesives, etc.
Used to synthesize anti-schistosomiasis drugs, as well as chemical sterilizers, fungicides and herbicides.



 微信扫一扫打赏
微信扫一扫打赏
