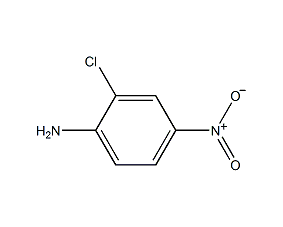
Structural formula
| Business number | 03E5 |
|---|---|
| Molecular formula | C6H5ClN2O2 |
| Molecular weight | 172.57 |
| label |
4-nitro-2-chloroaniline, p-nitro-o-chloroaniline, 2-Chloro-4-nitroaniline, o-Chloro-p-nitroaniline, 4-amino-3-chloronitrobenzene, 2-Chloro-4nitroaniline, 4-amino-3-chloronitrobenzene, 2-Chloro-4-nitroaniline, 98+%, 2-Chloro-4-Nitro-Anilin, 2-Chloro-4-Nitro-Benzenamin, Benzenamine,2-Chloro-4-Nitro, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:121-87-9
MDL number:MFCD00007665
EINECS number:204-502-2
RTECS number:BX1400000
BRN number:638657
PubChem number:24846548
Physical property data
1. Properties: yellow crystalline powder
2. Melting point (℃): 108
3. Solubility: soluble in ethanol, ether, benzene and carbon disulfide, slightly soluble in Water and strong acids are insoluble in naphtha.
Toxicological data
1. Acute toxicity: Rat oral LD50: 6430mg/kg
Mouse oral LD50: 1250mg/kg
Mouse peritoneal LDL0: 500mg/kg
Intravenous LD50 of mice: 56mg/kg
Oral LD50 of guinea pigs: 1800mg/kg
2. Mutagenicity: Salmonella mutation test system: 1mg/ plate
Ecological data
None
Molecular structure data
1. Molar refractive index: 41.92
2. Molar volume (cm3/mol): 115.5
3. Isotonic specific volume (90.2K): 324.4
4. Surface tension (dyne/cm): 62.2
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 16.62
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 71.8
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 159
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond unitsQuantity: 1
Properties and stability
This product is toxic. Its toxicity and protection methods are similar to those of o-nitro-p-chloroaniline.
Storage method
Packed in iron barrels or plastic bags, 50kg per barrel (bag). Store in a cool, ventilated place. Store and transport according to regulations on toxic substances.
Synthesis method
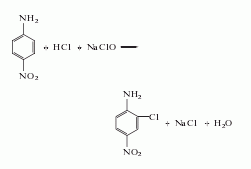
1. Chlorination method of p-nitroaniline: Add hydrochloric acid and sodium hypochlorite to chlorine p-nitroaniline. The reaction product is filtered, washed, and dried to obtain the finished product. Raw material consumption quota: p-nitroaniline 928kg/t, sodium hypochlorite (10%) 5600kg/t, 30% hydrochloric acid 5600kg/t.
2. Ortho-chloro to nitro Phenol method: obtained by ammoniation. Add 3.471g of o-chloro-p-nitrophenol (0.02mol), 20g of 28% ammonia water and 6.608g of ammonium sulfate (0.05mol) into a 50ml stainless steel autoclave, and react at 180°C for 7 hours. The conversion rate of o-chloro-p-nitrophenol is 50.9%, and the selectivity of o-chloro-p-nitroaniline is 98.2%.
Purpose
Intermediates of dyes and pigments, mainly used in the production of pigments vermilion R, disperse dyes red GFL, disperse red B, etc., and also used in the production of snail-killing pesticides. And used as pharmaceutical intermediates, such as preparing schistosomiasis-67 paste.



 微信扫一扫打赏
微信扫一扫打赏
