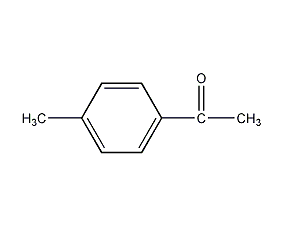
Structural formula
| Business number | 03ED |
|---|---|
| Molecular formula | C9H10O |
| Molecular weight | 134.18 |
| label |
p-acetyltoluene, 4-methylacetophenone, Methyl acetophenone, p-Acetyl-toluene, 4-Methylacetophenone, 1-(4-Methylphenyl)ethanone, food spices |
Numbering system
CAS number:122-00-9
MDL number:MFCD00008751
EINECS number:204-514-8
RTECS number:AM9463000
BRN number:606053
PubChem number:24901243
Physical property data
1. Properties: Colorless to slightly yellow transparent liquid, solidifies at a slightly lower temperature, with the aroma of hawthorn flowers and the aroma of alfalfa, honey and coumarin, and the aroma is softer than that of acetophenone , it has a sweet aroma like strawberry after extreme dilution.
2. Boiling point (ºC, 101.3kPa): 226
3. Boiling point (ºC, 1.5kPa): 113
4. Melting point (ºC): 28
5. Relative density (g/mL, 20/4ºC): 1.0051
6. Refractive index (n20ºC): 1.5335
7. Flash point (ºC): 92
8. Solubility: Easily soluble in ethanol, ether, chloroform and propylene glycol, etc., almost insoluble in water and glycerol.
9. Refractive index at room temperature (n25): 1.5315
10. Relative density (25℃, 4℃): 0.996630
Toxicological data
1. Acute toxicity: Rat oral LD50: 1400mg/kg, rabbit LD50: >2000mg/kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 41.10
2. Molar volume (cm3/mol): 137.2
3. Isotonic specific volume (90.2K ): 330.1
4. Surface tension (dyne/cm): 33.4
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 16.29
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 7
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 121
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Toxic. Avoid inhaling the vapor of this product and avoid contact with eyes and skin.
2. Exist in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves and smoke.
3. Naturally found in cocoa, blackcurrant, cheese, rosewood oil, Braziliansandalwood oil, Tibetan cypress oil, linoleum oil, And in mimosa.
Storage method
Store in a cool place.
Synthesis method
1. Mainly use acetylation method. It is obtained by using toluene and acetic anhydride as raw materials and performing an acetylation reaction in the presence of anhydrous aluminum trichloride catalyst, followed by hydrolysis, neutralization, water washing, separation and distillation. It can also be extracted from natural raw materials such as Brazilian sandalwood and rosewood through distillation.
2. Tobacco: BU, 56; OR, 57; FC, 9, 18.
Purpose
1. Used to blend flower essential oils; also used in the manufacture of soaps and fruity spices such as strawberries.
2. Commonly used in baked goods, candies, and puddings. It can be used in the formulation of daily chemical flavors and food flavors.



 微信扫一扫打赏
微信扫一扫打赏
