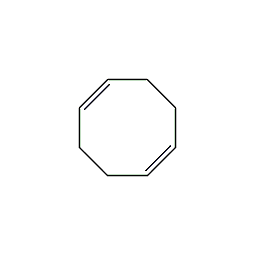
Structural formula
| Business number | 034A |
|---|---|
| Molecular formula | C8H12 |
| Molecular weight | 108.18 |
| label |
cis,cis-1,5-cyclooctadiene, 1,5-cyclooctadiene, Cis,cis-1,5-cyclooctadiene, 1,5-Cyclooctadiene, hydrocarbon solvents, Alicyclic compounds |
Numbering system
CAS number:111-78-4
MDL number:MFCD00001752
EINECS number:203-907-1
RTECS number:GX9620000
BRN number:2036542
PubChem number:24854773
Physical property data
1. Properties: colorless liquid
2. Boiling point (ºC, normal pressure): 150.9
3. Melting point (ºC): -70
4. Relative density (g/mL, 20/4ºC): 0.881
5. Refractive index (20ºC): 1.4933
6. Viscosity (mPa·s, 20ºC): 1.38
7. Viscosity (mPa·s, 50ºC): 0.87
8. Viscosity (mPa·s, 100ºC): 0.51
9. Specific heat capacity ( KJ/(kg·K), 50ºC): 2.010
10. Specific heat capacity (KJ/(kg·K), 100ºC): 2.180
11. Specific heat capacity (KJ/(kg ·K), 150ºC): 2.390
12. Thermal conductivity (W/(m·K), 30ºC): 0.1289
13. Heat of evaporation (KJ/mol, 101.3 kPa): 38.940
14. Flash point (ºC): 35
15. Fire point (ºC): 270
16. Vapor pressure (kPa, 20ºC ): 0.67
17. Solubility: Insoluble in water, insoluble in carbon tetrachloride.
Toxicological data
It has a strong irritating effect on mucous membranes and skin, causing skin allergies. There is no permanent harm to humans.
Ecological data
This substance is harmful to the environment, and special attention should be paid to atmospheric pollution.
Molecular structure data
1. Molar refractive index: 36.10
2. Molar volume (cm3/mol): 128.5
3. Isotonic specific volume (90.2K): 303.0
4. Surface tension (dyne/cm): 30.9
5. Polarizability (10-24cm3): 14.31
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: None
6. Topological molecular polaritySurface area 0
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 72.6
10 .The number of isotope atoms: 0
11. The number of determined atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 2
14. Uncertain number of chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
- No contact with strong oxidizing agents. Strictly avoid contact with skin and eyes during use, storage and transportation.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. should be kept away from oxidizer, do not store together. It should not be stored for a long time to avoid deterioration. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Should be stored in stainless steel bottles or plastic barrels in a cool, ventilated, dry warehouse, away from fire and heat sources. Store and transport according to general chemical regulations.
Synthesis method
Produced by the dimerization and cyclization of butadiene.


Purpose
It can also be used as an organic synthesis intermediate to prepare suberic acid, octenedioic acid, tetrachlorocyclooctane, etc. It can also be used as the third monomer of ethylene-propylene rubber.



 微信扫一扫打赏
微信扫一扫打赏
