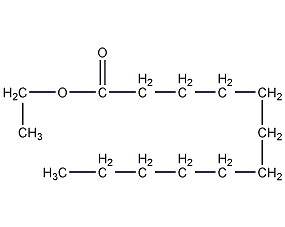
Structural formula
| Business number | 02T5 |
|---|---|
| Molecular formula | C14H28O2 |
| Molecular weight | 228.37 |
| label |
Ethyl laurate, Ethyl dodecanoate, Ethyl laurinate, Ethyl dodecanoate |
Numbering system
CAS number:106-33-2
MDL number:MFCD00015065
EINECS number:203-386-0
RTECS number:None
BRN number:1769671
PubChem ID:None
Physical property data
1. Properties: colorless liquid with mild fruity and floral aroma, and a slight oily smell.
2. Density (g/mL, 25℃): 0.862
3. Relative density (25℃, 4℃): 0.813086
4. Melting point (ºC): -10
5. Boiling point (ºC, normal pressure): 271
6. Boiling point (ºC, 3.33kPa): 163
7. Refractive index (D20): 1.4311
8. Flash point (ºC): >109
p>
9. Relative density (20℃, 4℃): 0.8618
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, 25ºC): 0.02
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil-water (octanol/water) partition coefficient relationship Value: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Insoluble in water, miscible in ethanol, chloroform, and ether.
Toxicological data
None yet
Ecological data
Generally speaking, it is not harmful to water bodies.
Molecular structure data
1. Molar refractive index: 68.68
2. Molar volume (cm3/mol): 263.1
3. Isotonic specific volume (90.2K ): 613.8
4. Surface tension (dyne/cm): 29.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 27.22
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 5.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 12
5. Number of tautomers: none
6. Topological molecule polar surface area 26.3
7. Number of heavy atoms: 16
8. Surface charge: 0
9. Complexity: 155
10. Number of isotope atoms:0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the chemical bond configuration Number of centers: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong acids, and strong alkali.
2. Found in flue-cured tobacco leaves.
3. Naturally found in cheese, blackberries, and wheat bread.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. It is produced by esterification of dodecanoyl chloride and ethanol in diethyl ether solution using magnesium as catalyst.
2. Tobacco: FC, 18. Produced by transesterification of ethanol and coconut oil esters in the presence of sulfuric acid.
Purpose
1. Used as flavors, fragrances, spandex additives and pharmaceutical raw materials, etc., used as gas chromatography stationary liquid, flavors, and also used in organic synthesis.
2. Widely used in daily chemical flavors, food flavors and wine flavor formulas.
3. Commonly used in baked goods, frozen dairy products, and puddings.



 微信扫一扫打赏
微信扫一扫打赏
