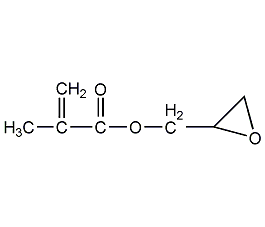
Structural formula
| Business number | 02U5 |
|---|---|
| Molecular formula | C7H10O3 |
| Molecular weight | 142.15 |
| label |
2,3-Epoxypropane methacrylate, Glycidyl methacrylate, methacrylic acid ring, 2,3-Methyl acrylate of propylene oxide, Glycidyl methacrylate, GMA, reactive diluent, fiber treatment agent, antistatic agent, Multifunctional solvent |
Numbering system
CAS number:106-91-2
MDL number:MFCD00005137
EINECS number:203-441-9
RTECS number:OZ4375000
BRN number:2506
PubChem number:24883127
Physical property data
1. Properties: Colorless and transparent liquid.
2. Relative density: 1.042
3. Melting point (ºC): -82
4. Boiling point (ºC): 189
p>
5 Boiling point (ºC, 1.33KPa): 75
6. Refractive index (20ºC): 1.4492
7. Flash point (ºC): 76
8. Autoignition point or ignition temperature (ºC): 389
9. Lower explosion limit (%, V/V): 1.1
10. Solubility: Yes Soluble in organic solvents, insoluble in water.
Toxicological data
1. Irritation: rabbit skin irritation: 500ul/24H severe irritation. Rabbit eye irritation: 100mg moderate intensity irritation.
2. Acute toxicity: Rat oral LD50: 500mg/kg; Mouse oral LD50: 390mg/kg;
Rabbit skin LD50: 480mg/kg; Rat Inhalation LC50: 45ppm/4H.
3. Irritating to skin and mucous membranes.
4. The LD50 of this product for intraperitoneal injection in mice: 1.122mg/kg, the oral LD50 of rats: 770mg/kg, and the LD50 of rabbit skin application: 450mg/kg.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 35.39
2. Molar volume (cm3/mol): 129.7
3. Isotonic specific volume (90.2K): 318.0
4. Surface tension (dyne/cm): 36.1
5. Dielectric constant:
6. Dipole moment (10-24 cm3):
7. Polarizability: 14.03
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.8
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 4
5. Number of tautomers: none
6. Topological molecule polar surface area 38.8
7 .Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 162
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
p>
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with acids, oxides, UV radiation, and free radical initiators. Soluble in almost all organic solvents and insoluble in water. This product is slightly toxic.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. Keep away from light. They should be stored separately from acids and oxidants, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Obtained from the reaction of sodium methacrylate and epichlorohydrin. Raw material consumption quota: methacrylic acid780kg/t, epichlorohydrin690kg/t, sodium hydroxide330kg/. It can also be obtained by the transesterification reaction of methyl methacrylate and glycidyl alcohol. Among them, the former method has easy-to-obtain raw materials and mild reaction, and is currently a commonly used method in the world. Process flow:(1) Preparation of methacrylic acid sodium salt Equipped with a stirrer and reflux In the four-necked bottle of the condenser, thermometer and dropping funnel, add 100ml of methacrylic acid, then add 100ml of solvent, stir evenly, and slowly add 103.5g of 50% NaOH aqueous solution dropwise. Control the reaction temperature not to exceed 55°C. After the dropwise reaction is completed, stir for 30 minutes, add 0.2g of polymerization inhibitor p-hydroxyanisole, dehydrate under reduced pressure at 85-90°C, 30.7-36.0KPa, dry to obtain sodium salt, seal and store for later use. 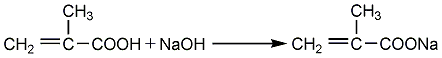 (2) GMA Synthesis In a three-necked flask equipped with a stirrer, water separator and thermometer, add 40g of dry methacrylic acid sodium salt, 0.2g of polymerization inhibitor p-hydroxyanisole, and 1.2g of catalyst (several quaternary ammonium salts, respectively). Generally use triethylbenzyl ammonium chloride) and 250g epichlorohydrin, stir the reaction, control the reaction temperature at 105-110°C, and the reaction time is 3.0h. After the reaction is completed, filter to remove NaCL, and the resulting filtrate is distilled under reduced pressure under the conditions of 80-80°C/6.7-80.KPa to distill out epichlorohydrin; and then distilled out under reduced pressure under the conditions of 100-105°C/1.1-1.6KPa ProductGMA.
(2) GMA Synthesis In a three-necked flask equipped with a stirrer, water separator and thermometer, add 40g of dry methacrylic acid sodium salt, 0.2g of polymerization inhibitor p-hydroxyanisole, and 1.2g of catalyst (several quaternary ammonium salts, respectively). Generally use triethylbenzyl ammonium chloride) and 250g epichlorohydrin, stir the reaction, control the reaction temperature at 105-110°C, and the reaction time is 3.0h. After the reaction is completed, filter to remove NaCL, and the resulting filtrate is distilled under reduced pressure under the conditions of 80-80°C/6.7-80.KPa to distill out epichlorohydrin; and then distilled out under reduced pressure under the conditions of 100-105°C/1.1-1.6KPa ProductGMA. 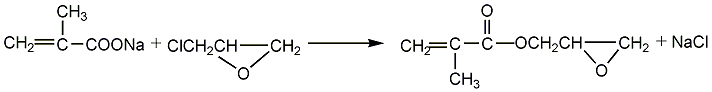
Purpose
1. Mainly used in powder coatings, but also in the bonding of thermosetting coatings, fiber treatment agents, adhesives, antistatic agents, vinyl chloride stabilizers, rubber and resin modifiers, ion exchange resins and printing inks. agent.
2.Used as functional monomer for polymerization reaction. Mainly used to manufacture acrylic powder coatings. It is used as a soft monomer to copolymerize with hard monomers such as methyl methacrylate and styrene. It can adjust the glass transition temperature and flexibility and improve the gloss, adhesion and weather resistance of the coating film. wait. Also used in the manufacture of acrylic emulsions and non-woven fabrics. As a functional monomer, it can be used to manufacture photosensitive resins, ion exchange resins, chelating resins, medical selective filtration membranes, dental materials, anticoagulants, solvent-free adsorbents, etc. It is also used to modify polyolefin resin, rubber and synthetic fibers.
3.Because its molecule contains both carbon-carbon double bonds and epoxy groups, it is widely used in polymer materials synthesis and modification. Used as reactive diluent for epoxy resin, stabilizer for vinyl chloride, modifier for rubber and resin, binder for ion exchange resin and printing ink. It is also used in powder coatings, thermosetting coatings, fiber treatment agents, adhesives, antistatic agents, etc. In addition, GMA also significantly improves the adhesion, water resistance, and solvent resistance of adhesives and non-woven coatings.
4. In electronics, it is used for photoresist films, electron beams, protective films, and far-infrared phase X-ray protective films. In terms of functional polymers, it is used in ion exchange resins, chelating resins, etc. In terms of medical materials, it is used in anti-blood coagulation materials, dental materials, etc.
Phase X-ray protective film. In terms of functional polymers, it is used in ion exchange resins, chelating resins, etc. In terms of medical materials, it is used in anti-blood coagulation materials, dental materials, etc.



 微信扫一扫打赏
微信扫一扫打赏
