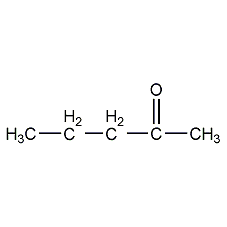
Structural formula
| Business number | 02VX |
|---|---|
| Molecular formula | C5H10O |
| Molecular weight | 86.13 |
| label |
Methyl propyl ketone, Ethyl acetone, Methyl Propyl Ketone, Methyl n-propyl ketone, Ethyl acetone, Excellent dewaxing agent for lubricants |
Numbering system
CAS number:107-87-9
MDL number:MFCD00009400
EINECS number:203-528-1
RTECS number:SA7875000
BRN number:506058
PubChem number:24870702
Physical property data
1. Properties: colorless liquid with acetone odor. [1]
2. Melting point (℃): -78[2]
3. Boiling point (℃): 101.7[3]
4. Relative density (water = 1): 0.81[4]
5. Relative vapor Density (air=1): 3.0[5]
6. Saturated vapor pressure (kPa): 4.7 (25℃)[6]
7. Heat of combustion (kJ/mol): -3099.4[7]
8. Critical temperature (℃): 290.8[8]
9. Critical pressure (MPa): 3.89[9]
10. Octanol/water partition coefficient: 0.91 [10]
11. Flash point (℃): 7 (CC) [11]
12. Ignition temperature (℃) ): 452[12]
13. Explosion upper limit (%): 8.2[13]
14. Explosion lower limit (%): 1.5[14]
15. Solubility: Slightly soluble in water, miscible in ethanol and ether. [15]
16. Heat of fusion (KJ/mol): 10.63
17. Volume expansion coefficient (K-1): 0.00111
18. Critical density (g·cm-3): 0.268
19. Critical volume (cm3 sup>·mol-1): 321
20. Critical compression factor: 0.253
21. Eccentricity factor: 0.346
22. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3137.65
23. Gas phase standard claimed heat (enthalpy) (kJ·mol– 1): -259.05
24. Gas phase standard entropy (J·mol-1·K-1): 378.7 p>
25. Gas phase standard formation free energy (kJ·mol-1): -138.0
26. Gas phase standard hot melt (J·mol– 1·K-1): 125.90
27. Liquid phase standard combustion heat (enthalpy) (kJ·mol-1) : -3099.41
28. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1): -297.29
29. Liquid phase standard entropy (J·mol-1·K-1): 274.1
30. Liquid phase standard formation free energy (kJ·mol– 1): -145.23
31. Liquid phase standard hot melt (J·mol-1·K-1): 184.2
Toxicological data
1. Acute toxicity[16]
LD50: 16000mg/kg (rat oral); 800mg/kg (rat intraperitoneal ); 1600mg/kg (orally for mice); 6500mg/kg (for rabbits through skin)
2. Irritation [17] Rabbit Transdermal: 405mg, mild irritation (open stimulation test)
3. Subacute and chronic toxicity[18] Mice inhaled 3g/m3, 5 hours a day, for 3 weeks, and no changes in body weight or blood were found.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradability No information available
Molecular structure data
1. Molar refractive index: 25.24
2. Molar volume (cm3/mol): 108.1
3. Isotonic specific volume (90.2K ): 236.1
4. Surface tension (dyne/cm): 22.6
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 10.00
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: 3
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 47.9
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[19] Stable
2. Incompatible substances[20] Strong oxidizing agent, strong reducing agent, strong alkali
3. Polymerization hazard[21] No polymerization
Storage method
Storage Precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 37°C. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents and alkalis, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
Refining method: After drying with barium oxide, use a 1m-long fractionating column to fractionate, and the distillate is treated with sodium bisulfite and refined. You can also use a distillation tower with a theoretical plate number of 100 to perform distillation at 101.3KPa and a reflux ratio of 100:1. The distillate is azeotropically distilled with water, and then dried with anhydrous calcium sulfate and distilled again. That is, pure product with a purity of 99.93%±0.01% can be obtained.
1. Obtained from the dehydrogenation of 2-sec-amyl alcohol.
![]()
2. From ethyl butyrylacetate Ester is obtained by heating together with water.
![]()
3. Preparation method:
p>
![]()
Refer to the above preparation method of 3-pentanone (Reference Book 335), react with 176g (2.0mol) of n-butyric acid (2) and 360g (6.0mol) of glacial acetic acid to obtain 75g of 2-pentanone (1), bp 102~104℃, yield 43%. 75g of acetone was obtained, bp 56~57°C. [24]
4. Preparation method:
In a dry reaction bottle equipped with a magnetic stirrer, reflux condenser, dropping funnel, and ventilation tube, add 3.9g (0.1mol) of metallic sodium, add dry nitrogen, and add 2,6- A solution of 24.7g (120mmol) of di-tert-butylphenol dissolved in 200mL of dry THF. Subsequently, the reaction proceeded vigorously, and the mixture was refluxed for 1 h to complete the reaction. Cool to 0°C and add 14.2 mL (100 mmol) of triethylboron. Add dropwise a solution of bromoacetone (2) 13.7g (100mmol) dissolved in 50mLTHF, stir the reaction for 30min, raise to room temperature, add 25mL ethanol, and isolate the product. Gas-liquid distribution chromatography analysis proved that the yield was 88%. Fractionate distillate and collect the fractions at 101-102°C to obtain 6.0g of compound (1) with a yield of 70%. [25]
Purpose
Mainly used as a solvent and also as a perfume. [23]



 微信扫一扫打赏
微信扫一扫打赏
