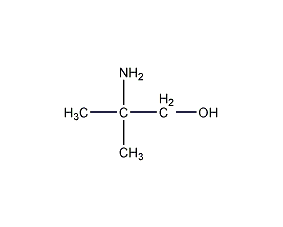
Structural formula
| Business number | 03JF |
|---|---|
| Molecular formula | C4H11NO |
| Molecular weight | 89.14 |
| label |
2-Aminoisobutanol, butylaminol, Isobutanolamine, β-Aminoisobutyl aroma, β-Aminoisobutyl alcohol, AMP, β-Amino-iso-butyl alcohol, 2,2-Diethylethanolamine, 2-Hydroxymethyl-2-propylamine, 2-Methyl-2-amino-1-propanol, aliphatic compounds |
Numbering system
CAS number:124-68-5
MDL number:MFCD00008051
EINECS number:204-709-8
RTECS number:UA5950000
BRN number:505979
PubChem number:24862008
Physical property data
1. Properties: Colorless and transparent liquid, or white petroleum jelly-like substance with a special smell.
2. Density (g/mL, 25/4℃): 0.934
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 30-31
5. Boiling point (ºC, normal pressure): 165
6. Boiling point (ºC, 1.33kPa): 67.4
7. Refractive index (n20): 1.448
8. Flash point (ºC): 67
9. Specific rotation Degree (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC) : Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Can be compared with Miscible in water and soluble in alcohol.
Toxicological data
Acute toxicity data
Rat oral LD50: 2900 mg/kg
Mouse oral LD50: 2150 mg/kg
Rabbit oral LDLo: 1mg/kg
Other multiple dose data
Rat oral TDLo: 44800 mg/kg/8W-C
Rat inhalation TCLo: 230 ug/m3/4H/13W-I
Dog oral TDLo: 11340 mg/kg/28D-C
Primate-monkey inhaled TCLo: 6 mg/m3/ 89D-I
Rodent-HamsterInhalation TCLo: 100 mg/m3/4H/13W-I
Ecological data
None
Molecular structure data
1. Molar refractive index: 25.61
2. Molar volume (cm3/mol): 95.7
3. Isotonic specific volume (90.2K ): 232.1
4. Surface tension (dyne/cm): 34.4
5. Polarizability (10-24cm3): 10.15
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.8
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 46.2
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 42.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Irritating to eyes and skin.
Storage method
Stored in a cool, dry and dark place.
Synthesis method
1. React 2-nitropropane with formaldehyde aqueous solution, and then concentrate it into an aqueous solution containing 70% 2-methyl-2-nitro-1-propanol. Dilute to 30% with methanol. Add to the autoclave, add 5% skeleton nickel by weight of nitro, and hydrogenate and reduce at 30°C and 267 atmospheric pressure for 6-8 hours. After distilling off methanol and most of the water under normal pressure, distill under reduced pressure to collect the 103°C (8.53-8.67kPa) fraction, which is 2-amino-2-methyl-1-propanol. Based on 2-nitropropane, the yield is 70.5%.
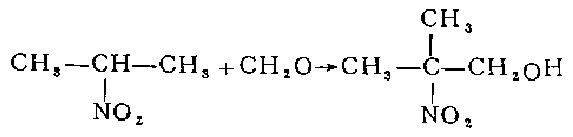
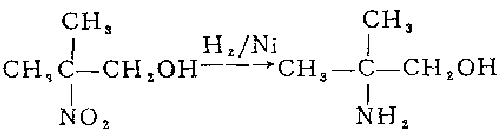
Purpose
1. Used to synthesize surfactants, vulcanization accelerators, and acid gas absorbers. Derivatives formed with carboxylic acid compounds are used in gas chromatography analysis.
2.Carbonyl protecting reagent. Synthesize 2,2-dimethylaziridine (a very useful intermediate) with high yield. Pine vinegar is the only highly efficient precipitant for L-piramic acid.
3. It is an organic base used as a neutralizing agent and is generally used as a neutralizing agent for resins in cosmetics.



 微信扫一扫打赏
微信扫一扫打赏
