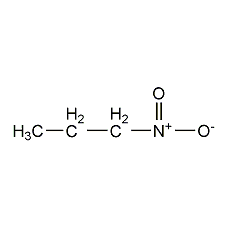
Structural formula
| Business number | 02W9 |
|---|---|
| Molecular formula | C3H7NO2 |
| Molecular weight | 89.09 |
| label |
propyl nitrate, propyl nitrate, Rubber and plastic additives, Nitrogen-containing compound solvent |
Numbering system
CAS number:108-03-2
MDL number:MFCD00007407
EINECS number:203-544-9
RTECS number:TZ5075000
BRN number:506236
PubChem number:24886609
Physical property data
1. Properties: colorless and transparent liquid with fruity aroma. [1]
2. pH value: 6.0 (0.01mol/L aqueous solution) [2]
3. Melting point (℃): -108.0[3]
4. Boiling point (℃): 131.6[4]
5. Relative density (water=1): 0.993[5]
6. Relative vapor density (air=1): 3.1[6]
7. Saturated vapor pressure (kPa): 1.00 (20℃)[7]
8. Heat of combustion (kJ/mol): -2011.96[8]
9. Critical temperature (℃): 402.0[9]
10. Critical pressure (MPa): 4.35 [10]
11. Octanol/water partition coefficient: 0.87[11]
12. Flash point (℃ ): 36 (CC); 34 (OC) [12]
13. Ignition temperature (℃): 421[13]
14. Explosion upper limit (%): 13.8[14]
15. Explosion lower limit (%): 2.2[15]
16. Solubility: Slightly soluble in water, soluble in chloroform, miscible in organic solvents such as ethanol and ether. [16]
Toxicological data
1. Acute toxicity[17]
LD50: 455mg/kg (rat oral)
LC50 : 3100ppm (rat inhalation, 8h)
2. Irritation No data available
3. Mutagenicity[18 ] Unprogrammed DNA synthesis: rat liver 1μmol/L. Sister chromatid exchange: Hamster ovary 74mg/L (24h)
4. Others[19] TCLo: 150ppm (human inhalation)
p>
Ecological data
1. Ecotoxicity[20] LC50: 205mg/L (48h) (zebrafish, static)
2. Biodegradability [21] Sealed bottle test, using municipal sewage treatment plant sludge, degraded 45% in 28 days.
3. Non-biodegradability[22] In the air, when the hydroxyl radical concentration is 5.00×105 pcs/cm3, the degradation half-life is 37 days (theoretical).
Molecular structure data
1. Molar refractive index: 21.97
2. Molar volume (cm3/mol): 90.8
3. Isotonic specific volume (90.2K ): 210.0
4. Surface tension (dyne/cm): 28.5
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 8.70
Compute chemical data
1.Sparse� Reference value for parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4 .Number of rotatable chemical bonds: 1
5. Number of tautomers: 2
6. Topological molecule polar surface area 45.8
7. Heavy atoms Number: 6
8. Surface charge: 0
9. Complexity: 47.2
10. Number of isotope atoms: 0
11. Determine the number of stereocenters of atoms: 0
12. Determine the number of stereocenters of atoms: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties Aqueous solution is acidic, pH of 0.01mol/L aqueous solution is 5.61; pH of saturated aqueous solution of nitropropane is 4.33; pH of water-saturated nitropropane is 4.06.
2. Nitropropane is more toxic than nitromethane and nitroethane, while 1-nitropropane is more toxic than 2-nitropropane, and both are locally irritating. It is very strong and very toxic to the liver and kidneys. All experimental animals can be killed by gases in concentrations above 1%. For protective methods, see Nitroethane.
3. Stability[23] Stable
4. Incompatible substances[24] Strong reducing agents, inorganic bases, alkali metals, halogenated alkanes, metal hydrides, metal alkoxides, ammonia, amines, etc.
5. Conditions to avoid contact [25] Strong vibration, heat
6. Polymerization hazard[26] No polymerization p>
7. Decomposition products[27] Nitrogen oxides
Storage method
Storage Precautions[28] Store in a cool, ventilated warehouse. The storage temperature should not exceed 37°C. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from oxidants, acids, alkalis, etc. and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from nitration of propane. First, propane is placed in a preheater at 430-450°C for preheating, and then introduced into a reaction tower lined with glass or silica. There are several places in the reaction tower where 75% nitric acid can be injected into the internal propane gas. The reaction temperature is adjusted to 390-440°C, the pressure is 0.69-0.86MPa, and the molar ratio of propane to nitric acid is 5:1. The gas coming out of the reaction tower is The condenser cools, and nitropropane and dilute nitric acid are condensed. The propane and gaseous oxides are recovered by the recovery tower, and the propane is recycled. The obtained products are 10%-30% nitromethane, 20%-25% nitroethane, 25% 1-nitropropane, and 40% 2-nitropropane. This product can also be obtained by nitration of propylene.
2. Nitropropane can also be produced from unsaturated hydrocarbons such as propylene through gas phase or liquid phase nitration, or liquid phase nitration of saturated hydrocarbons.
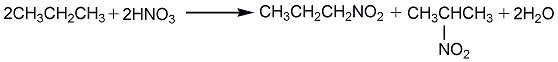
Purpose
1. Mainly used as solvents and intermediates, jet fuel, sprays, etc. (1) As a solvent, it has strong dissolving power for alcohols, ketones, ethers, esters, as well as dyes, greases, waxes, resins, and synthetic rubber. Used together with alcohol, it is a powerful solvent for acetate fiber; used together with alcohol and mandarin, it can replace chlorinated hydrocarbon solvents to dissolve triacetate fiber. As a low-temperature solvent, it can dissolve vinyl chloride-vinyl acetate copolymer and can also be used to dissolve nitrocellulose. (2) It is an intermediate for chemical products such as amines, hydroxylamines, nitrohydroxy compounds, chlorinated nitroalkanes, etc. For example, nitropropane and methane are hydrolyzed in the presence of sulfuric acid to obtain hydroxylamine sulfate and propionic acid. It is used in the pharmaceutical industry to produce the anti-tuberculosis drug ethambutyl hydrochloride.
2. This product is an excellent solvent for various cellulose derivatives, vinyl resins, especially vinyl copolymers, nitrocellulose paint, synthetic rubber, grease, dyes, etc. Mixed solvents composed of this product and other solvents are widely used in the coating industry and adhesive industry. Also used in pharmaceutical and organic synthesis intermediates.
3. Used in organic synthesis, and as solvents, rocket propellants, gasoline additives, etc. [29]



 微信扫一扫打赏
微信扫一扫打赏
