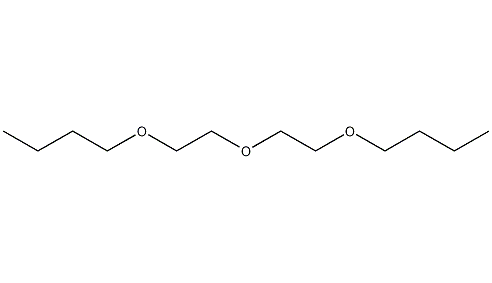
Structural formula
| Business number | 036J |
|---|---|
| Molecular formula | C12H26O3 |
| Molecular weight | 218.33 |
| label |
2,2′-dibutoxyethyl ether, Diethylene glycol dibutyl ether, diethylene glycol dibutyl ether, 1-[2-(2-Butoxyethoxy)ethoxy]butane, 2-Butoxyethyl ether, Dibutyl carbitol, Diethylene glycol dibutyl ether, solvent coupling agent, Extracting agent, Thinner |
Numbering system
CAS number:112-73-2
MDL number:MFCD00009459
EINECS number:204-001-9
RTECS number:KN0350000
BRN number:1750713
PubChem number:24852213
Physical property data
1. Properties: colorless liquid with slight ether smell.
2. Relative density (g/mL, 20/20℃): 0.8853
3. Relative vapor density (g/mL, air=1): >1
4. Freezing point (ºC): -60.2
5. Boiling point (ºC, normal pressure): 256
6. Refractive index (20ºC): 1.4233
p>
7. Viscosity (mPa·s, 20ºC): 2.4
8. Flash point (ºC): 118
9. Fire point (ºC): 310
p>
10. Vapor pressure (kPa, 20 ºC): 0.0013
11. Vapor pressure (kPa, 122ºC): 1.33
12. Vapor pressure (kPa, 162ºC ): 6.67
13. Heat of evaporation (KJ/mol): 56.1
14. Liquid phase standard hot melt (J·mol-1·K -1): 463.4
15. Critical pressure (KPa): Undetermined
16. Specific heat capacity (KJ/(kg·K), 13ºC, Constant pressure): 1.80
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Soluble in alcohol, 0.3% dissolved in water at 20°C; 1.4% dissolved in water in diethylene glycol dibutyl ether. Can dissolve grease, rosin, glycerol trirosinate, chloroprene rubber, hydrocarbons, etc. It forms an azeotropic mixture with 94.7% water, with an azeotropic point of 99.8°C.
20. Relative density (20℃, 4℃): 0.8850
21. Refractive index at room temperature (n20): 1.4235
Toxicological data
1. Irritation: Rabbit transdermal: open irritation test, 500mg, mild irritation. Rabbit eye: 500mg/24 hours, mild irritation.
2. Acute toxicity: rat oral LD50: 3900mg/kg; rabbit dermal LD50: 5400ul/kg
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water pollution.
Molecular structure data
1. Molar refractive index: 62.87
2. Molar volume (cm3/mol): 245.7
3. Isotonic specific volume (90.2K ): 568.5
4. Surface tension (dyne/cm): 28.6
5. Polarizability (10-24cm3): 24.92
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 12
5. Number of tautomers: none
6. Topological molecule polar surface area 27.7
7. Number of heavy atoms: 15
8. Surface charge: 0
9. Complexity: 95
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Avoid contact with strong oxidizing agents. It is non-corrosive to metals and can generate peroxide under the action of oxygen in the air during storage.
Chemical properties: Has the chemical properties of ether.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Should be sealed and stored away from light. should be kept away from oxidizer, do not store together. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials. Can be stored in iron, mild steel, copper or aluminum containers.
Synthesis method
Industrial production uses ethylene oxide and n-butanol as raw materials, or the reaction of diethylene glycol and n-butanol.
Refining method: Slowly pass diethylene glycol dibutyl ether through a column filled with activated alumina to remove peroxide. The effluent is shaken with sodium carbonate to remove acidic impurities. Then wash with water, dry with calcium chloride and fractionate under reduced pressure.
Purpose
This product is an industrial solvent, mainly used as a dilute aqueous solution extraction agent for fatty acids, for the extraction of uranium, polonium, and gold. It is also used in the spice and pharmaceutical industries. It can also be used as a solvent coupling agent and hydraulic fluid medium. It is used as a diluent for polyvinyl chloride latex, and is also used in the separation and refining of alkyl phosphoric acid, uranium ore extraction, pharmaceutical industry, etc.



 微信扫一扫打赏
微信扫一扫打赏
