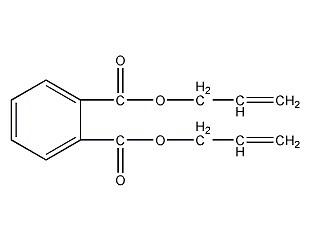
Structural formula
| Business number | 03MV |
|---|---|
| Molecular formula | C14H14O4 |
| Molecular weight | 246.27 |
| label |
plasticizer, cross-linking agent, Aromatic carboxylic acids and their derivatives |
Numbering system
CAS number:131-17-9
MDL number:MFCD00008646
EINECS number:205-016-3
RTECS number:CZ4200000
BRN number:1880877
PubChem number:24862810
Physical property data
1. Properties: colorless or light yellow oily liquid. Mild odor, flammable and tear-inducing.
2. Density (g/mL, 20/4℃): 1.1099
3. Relative vapor density (g/mL, air=1): Undetermined
4 . Melting point (ºC): -70
5. Boiling point (ºC, normal pressure): 290
6. Boiling point (ºC, 0.53kPa): 165~167 (666.5pa)
7. Refractive index (n20D): 1.520 (25℃)
8. Flash point (ºC): >110
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): 181
p>
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ /mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Oil and water Log value of (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V /V): Undetermined
19. Solubility: Insoluble in water, slightly soluble in petroleum ether and glycerin, miscible with various organic solvents such as ethanol, benzene and ethyl acetate.
Toxicological data
1. Acute toxicity:Oral – Rat LD50: 656 mg/kg 2. Stimulation data:Eye-rabbit 500 mg mild
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 67.78
2. Molar volume (cm3/mol): 221.4
3. Isotonic specific volume (90.2K ): 554.1
4. Surface tension (dyne/cm): 39.2
5. Polarizability (10-24cm3): 26.87
Calculate chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 8
5. Number of tautomers: none
6. Topological molecule polar surface area 52.6
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 290
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Insoluble in water, soluble in organic solvents such as ethanol, ether, acetone, benzene, and partially soluble in mineral oil, glycerin, and ethylene glycol. It has good compatibility with polymers such as cellulose acetate, polystyrene, vinyl acetate copolymer and polymethyl methacrylate. Flammable, low toxicity.
2.This product has low toxicity, but can irritate mucous membranes and skin and cause dermatitis. Contact with skin and mouth should be avoided. The production process should be sealed and operators should wear protective equipment.
3. Exist in tobacco leaves.
Storage method
Stored in a cool, dry warehouse, away from fire sources and strong oxidants, protected from sun and rain.
Packaged in galvanized iron drums. Store away from light, away from fire sources, in a cool and ventilated place.
Synthesis method
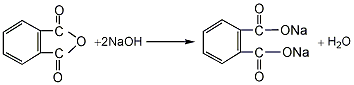
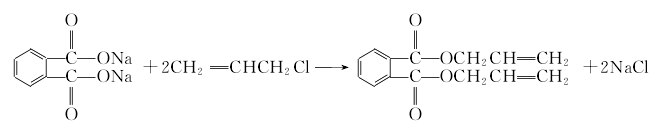
1. Phthalic anhydride reacts with liquid alkali to form phthalic acid sodium salt, which is then esterified with propylene chloride at 40-60°C under normal pressure to obtain the crude product. After filtration, neutralization, water washing and vacuum distillation, the finished product is obtained. Raw material consumption quota: phthalic anhydride (≥90%) 780kg/t, propylene chloride (≥95) 1060kg/t, liquid alkali (40%) 1080kg/t.
2. In ester Add 119kg of phthalic anhydride, 119g of hydroquinone, 121kg of propylene alcohol, 156kg of benzene and 5.47kg of 65% sulfuric acid into the pot. The reaction is heated, and the temperature rises uniformly from 80°C to 95°C. The benzene and water generated by the reaction are azeotropically evaporated, and the water is removed through condensation and stratification. Benzene was continuously refluxed. When the water output per hour was less than 100 mL, the reaction was terminated and cooled. Put the product into the neutralization washing pot, add 68kg of benzene, add 200kg of water or 100kg of 5% alkali solution for alternate neutralization and washing, stir for 15 minutes each time, let it stand for stratification, drain the waste water and wash again until the pH value of the water layer for neutral. Then add the neutral alcoholate to the dealcoholization pot, steam out the benzene at 80-140°C, and then distill under reduced pressure. The 155-165°C fraction is collected as the finished product. The esterification reaction formula is as follows

Purpose
1. This product is a reactive plasticizer, mainly used to prepare diisopropyl phthalate resin, as a cross-linking agent for unsaturated polyester resin and as a reinforcing agent for cellulose resin. and used as plasticizers for resins that can self-polymerize without the addition of inhibitors.
2. It can be used as a plasticizer for resins that are easy to self-polymerize without adding inhibitors. It can also be used as a cross-linking agent for diisopropyl phthalate resin (DAP), unsaturated polyester resin, and a plasticizer for cellulose resin.
3. Used as a cross-linking agent for unsaturated polyester resin. It can also be used as a reactive plasticizer for polymerizable plasticizers such as vinyl resin, cellulose resin, polyvinyl chloride, and resins that polymerize by themselves without adding inhibitors. It is also used as a comonomer for various monomers and unsaturated compounds, a catalyst for polyester resins, a cross-linking agent for unsaturated polyester resins, a reinforcing agent for cellulose resins, and in the manufacture of polydiallyl phthalate. Resin and used as pigment carrier, etc.



 微信扫一扫打赏
微信扫一扫打赏
