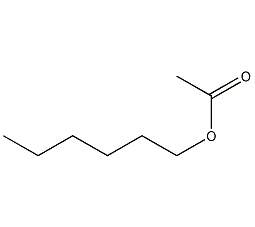
Structural formula
| Business number | 03VM |
|---|---|
| Molecular formula | C8H16O2 |
| Molecular weight | 144.21 |
| label |
n-hexyl acetate, n-hexyl acetate, n-hexyl acetate, n-Hexyl acetate, Hexyl ethanoate, Acetic acid n-hexyl ester, food additives, Flavor enhancer |
Numbering system
CAS number:142-92-7
MDL number:MFCD00009524
EINECS number:205-572-7
RTECS number:AI0875000
BRN number:1747138
PubChem number:24893086
Physical property data
1. Properties: Colorless oily liquid with pleasant fruit aroma and pear-like sweet and sour taste.
2. Density (g/mL, 20/4℃): 0.8779
3. Relative vapor density (g/mL, air=1): 4.97
4. Melting point (ºC): -80
5. Boiling point (ºC, normal pressure): 168~171.5
6. Flash point (ºC): 37
7. Refractive index (n20ºC): 1.4093
8. Solubility: Miscible with ethanol, ether, benzene and other solvents, insoluble in water.
9. Relative density (25℃, 4℃): 0.8679
10. Refractive index at room temperature (n25): 1.4073
11. Critical temperature (ºC): 345.25
12. Solubility parameter (J·cm-3)0.5: 17.197
13. van der Waals area (cm2·mol-1): 1.319×1010
14. van der Waals volume (cm3·mol-1): 93.690
15. Liquid phase standard hot melt (J·mol-1·K-1): 297.2
Toxicological data
1. Acute toxicity: rat oral LD50: 41500 uL/kg; rabbit dermal LD50: >5 gm/kg.
2. Toxic or toxic vapor. May be harmful to the body if inhaled, ingested, or absorbed through the skin.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 40.88
2. Molar volume (cm3/mol): 164.0
3. Isotonic specific volume (90.2K ): 375.1
4. Surface tension (dyne/cm): 27.3
5. Polarizability (10-24cm3): 16.20
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 2.4
2. Hydrogen bond supply�Number: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 6
5. Topological molecule polar surface area ( TPSA): 26.3
6. Number of heavy atoms: 10
7. Surface charge: 0
8. Complexity: 89.3
9. The number of isotope atoms: 0
10. The number of determined atomic stereocenters: 0
11. The number of uncertain atomic stereocenters: 0
12. Determined number of stereocenters of chemical bonds: 0
13. Uncertain number of stereocenters of chemical bonds: 0
14. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure. Incompatible materials: strong oxidants, strong acids, strong reducing agents, strong bases.
2. It is easy to cause combustion when exposed to high heat, open flames or strong oxidants.
3. Found in flue-cured tobacco leaves.
Storage method
Keep sealed in a cool place.
Synthesis method
1. Obtained from the esterification of acetic acid and hexanol. Add 23kg n-hexanol, 15kg acetic acid, 18kg benzene, and 250g sulfuric acid into the glass-lined reaction pot. Slowly heat and reflux, and the steamed product is condensed in the oil-water separator to separate benzene and refluxed into the reaction pot. After about 2 hours, when the refluxing benzene becomes clear, the moisture has been removed. Continue to steam out benzene and recycle it for reuse. The residue is a crude product, which is washed several times with 5% sodium carbonate solution and then washed with water. After drying with anhydrous potassium carbonate, carry out fractionation, and collect the 170-173°C fraction to obtain the finished product.
2. Obtained from the boiling reaction of n-hexanol and excess acetic anhydride (or excess acetic acid in the presence of concentrated sulfuric acid).
3. Tobacco: FC, 40.
Purpose
GB 2760-96 stipulates the edible spices allowed to be used. Mainly used for preparing fruit flavors such as apples and pears. Ethyl acetate has a fruity aroma and is used in edible fruit flavors. Also used in organic synthesis. Cellulose esters, resins and other solvents.



 微信扫一扫打赏
微信扫一扫打赏
