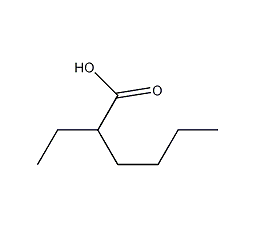
Structural formula
| Business number | 03XU |
|---|---|
| Molecular formula | C8H16O2 |
| Molecular weight | 144.21 |
| label |
isooctanoic acid, subcaprylic acid, Butylacetic acid, 2-Butylhexanoic acid, Heptane-3-carboxylic acid, Ethyl acetic acid, Butylethylacetic acid, Ethylhecoic acid, 2-Butylbutanoic acid, acid solvents, aliphatic compounds |
Numbering system
CAS number:149-57-5
MDL number:MFCD00002675
EINECS number:205-743-6
RTECS number:MO7700000
BRN number:1750468
PubChem number:24878352
Physical property data
1. Properties: colorless oily liquid.
2. Boiling point (ºC, 101.3kPa): 227.6
3. Melting point (ºC): -59
4. Relative density (g/mL, 20/4ºC): 0.9077
5. Relative density (g/mL, 25/4ºC): 0.9031
6. Relative vapor density (g/mL, air=1): 4.9
7. Refractive index (20ºC): 1.4252
8. Refractive index (25ºC): 1.4287
9. Viscosity (mPa·s, 20ºC) : 7.73
10. Flash point (ºC, open): 114
11. Fire point (ºC): 371
12. Vapor pressure (kPa, 20ºC ): 0.0040
13. Vapor pressure (kPa, 106ºC): 0.67
14. Lower explosion limit (%, V/V): 0.8
15. Explosion upper limit (%, V/V): 6.0
16. Volume expansion coefficient (K-1, 20ºC): 0.00089
17. Solubility : Slightly soluble in cold water and ethanol, soluble in hot water, ether, ethyl acetate, acetic acid, acetone, benzene and chloroform. Dissolves 0.25% in water at 20°C; water dissolves 1.2% in 2-ethylhexanoic acid.
18. Critical temperature (ºC): 419.85
19. Critical pressure (MPa): 2.78
20. Critical density (g·cm -3): 0.273
21. Critical volume (cm3·mol-1): 528
22. Critical compression factor: 0.262
23. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -4875.18
24. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -559.54
25. Liquid phase standard heat of combustion (enthalpy) (kJ·mol-1 ): -4799.58
26. The liquid phase standard claims heat (enthalpy) (kJ·mol-1): -635.14
Toxicological data
1. Acute toxicity: Rat oral LD50: 3 mg/kg; rabbit dermal LD50: 1260 uL/kg.
2. Inhalation toxicity: Rat: >400 ppm/6H
3. Reproductive toxicity: Rat oral TDLo: 1803 mg/kg; Rat oral TDLo: 5 mg/kg.
4. It is of low toxicity. Irritating to skin and mucous membranes.
Ecological data
This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 40.63
2. Molar volume (cm3/mol): 155.5
3. Isotonic specific volume (90.2K ): 369.6
4. Surface tension (dyne/cm): 31.8
5. Polarizability (10-24cm3): 16.10
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 5
5. Number of tautomers: none
6. Topological molecule polar surface area 37.3
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 99.4
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable under normal temperature and pressure.
Incompatible materials: strong oxidizing agents
2.This product has low toxicity. Irritating to respiratory tract and mucous membranes. Rats were given intragastric administration and it was found that congestion of internal organs, liver dystrophy and multiple necrosis of kidneys were found. Rats were given LD503000mg/kg orally, and mice were given LD501120mg/kg intravenously. The equipment should be sealed and operators should wear masks and protective glasses.
3. Exists in flue-cured tobacco leaves, burley tobacco leaves, oriental tobacco leaves, and mainstream smoke.
Storage method
1. Store sealed in a dry and cool place.
2. Packed in iron drums or galvanized iron drums lined with plastic bags, net weight 180kg. It can also be packed in plastic barrels with a net weight of 25kg. Store and transport according to general chemical regulations.
Synthesis method
Some foreign companies use butyraldehyde as raw material and obtain 2-ethylhexenal through condensation and dehydration.
1.-Ethylhexanol oxidation method: It is prepared by oxidizing 2-ethylhexanol in an aqueous sodium hydroxide solution with potassium permanganate to form sodium isooctanoate, and then neutralizing it with sulfuric acid. Raw material consumption quota: 2-ethylhexanol 1204kg/t, potassium permanganate 3611kg/t, liquid caustic soda approximately 1500kg/t, sulfuric acid approximately 960kg/t.
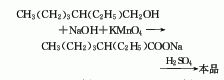
2.-Ethylhexene The aldehyde oxidation method uses 2-ethylhexenal, an intermediate product of propylene carbonylation to produce 2-ethylhexanol, as raw material, 2-ethylhexenal is produced through selective hydrogenation, and then 2-ethylhexenal is produced through liquid phase oxidation. Hexanol. 3. Catalytic dehydrogenation esterification method of 2-ethylhexanol. Under alkaline conditions, using cadmium oxide, zinc oxide, and manganese dioxide as catalysts, 2-ethylhexanol is dehydrogenated to 2-ethylhexanol at 180-210°C. Acid ester (isooctanoate), isooctanoate is saponified to generate the corresponding salt and alcohol. After the salt is acidified with sulfuric acid, it is then distilled to obtain the finished product of isooctanoic acid.
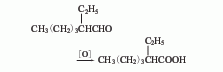
3. Tobacco: OR, 18 ; BU, 26; FC, 40.
4. Preparation method:
Add 100mL of water and 2.0kg of sodium hydroxide into the reaction kettle, stir and dissolve. Add 5.0kg (38.5mol) of isooctyl alcohol (2) under constant stirring. Control the reaction temperature to 20°C and add 16.0kg (95mol) of potassium permanganate in batches. Raise the temperature to 40-50°C and stir for 4 hours until the purple-red color of potassium permanganate disappears. Filter and wash the filter cake with hot water. Concentrate under reduced pressure to 1/2 of the original volume. The filtrate was adjusted to pH 1-2 with dilute sulfuric acid. Separate the oil layer, distill under reduced pressure, and collect the 110~130℃/2.66kPa fraction. Obtain isooctanoic acid (1) ① 4.4kg, with a yield of 79%. Note: ① It can also be prepared by nitric acid oxidation of isooctyl alcohol, with a yield of 35%. [1]
Purpose
Most of 2-ethylhexanoic acid is converted into salts of metal zirconium, cobalt, molybdenum, zinc, etc., which are used as paint driers and heat stabilizers for polyvinyl chloride plastics; tin salts are used as additives for plastic pipes; barium Salt and cadmium salt are used in plastic calendered products and stabilizers. 2-ethylhexanoic acid and its esters are also used in medicine, fungicides, metal lubricants, cosmetics, etc. Its glyceride is an excellent plasticizer. 2-Ethylhexanoic acid is the raw material of the pharmaceutical carbenicillin. It is also used in the synthesis of many dyes and spices.



 微信扫一扫打赏
微信扫一扫打赏
