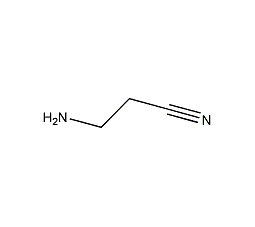
Structural formula
| Business number | 03YP |
|---|---|
| Molecular formula | C3H6N2 |
| Molecular weight | 70.09 |
| label |
β-Aminopropionitrile, β-amine propionitrile, beta-Cyanoethylamine, N-(2-Cyanoethyl)amine |
Numbering system
CAS number:151-18-8
MDL number:MFCD00014820
EINECS number:205-786-0
RTECS number:UG0350000
BRN number:1698848
PubChem ID:None
Physical property data
1. Physical property data:
1.Characteristics: This product is liquid.
2 .Refractive index (nD20): 1.4369
3.Density (g/mL): 0.9584
4 .Boiling point (ºC):185
5 .Boiling point (ºC,2.67kpa):87-89
6 .Boiling point (ºC,0.67kpa):66-69
7 .Boiling point (ºC,0.4kpa):50-55
8.Flash Point (ºC):79-81ºC
9.Saturated vapor pressure (kPa,38 -40ºC): 0.27
Toxicological data
2. Toxicological data:
1, acute toxicity: mouse transdermal LDLo: 12800 mg/kg;
Mouse abdominal cavity LD50: 1152 mg/kg.
2. Reproductive toxicity: Rat oral TDLo: 5 gm/kg ;
Mouse abdominal cavity TDLo: 1250 mg/kg.
Ecological data
3. Ecological data:
Other harmful Effect: This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
5. Molecular property data: 1. Molar refractive index:19.39 2. Molar volume (m3 /mol):75.1 3. Isotonic specific volume (90.2K): 188.8 4. Surface tension (dyne/cm): 39.9 5. Polarizability(10-24cm3):7.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 49.8
7. Number of heavy atoms: 5
8. Surface charge: 0
9. Complexity: 49.2
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Stable under normal temperature and pressure.
Incompatible materials:Strong oxidants, strong acids, strong bases.Storage method
Save in a dry and cool place
Synthesis method
Obtained from the amination of acrylonitrile. Cool the dry autoclave to 35℃ or below, add acrylonitrile, diphenylamine, and tert-butyl alcohol in sequence, stir 5min, Add liquid ammonia, control the temperature at 100-109℃, the pressure at about 1MPa, keep warm and stir 4h. Cool to below 10℃ and stop stirring when the pressure drops to normal pressure. Recover tert-butanol under reduced pressure at 65-70℃ (14.7-21.3kPa) to obtain crude product 3-Aminopropionitrile, the crude product is distilled under reduced pressure and collected 66-105℃ (1.33-4.0kPa) fraction to obtain 3- aminopropionitrile.
Purpose
Used as organic synthesis and pharmaceutical intermediates.
Intermediate for the synthesis of alanine and pantothenic acid; can be obtained by reduction1,3-Diaminopropane; reacts with phosgene to produce isocyanate, which can further react with p-nitroaniline to produce a sweetener that is sweeter than sugar350 times; the hydrogen on ammonia can be substituted by aliphatic hydrocarbons, alicyclic hydrocarbons, aromatic hydrocarbons and heterocyclic rings to generate Corresponding alkylaminopropionitrile;
ONT-SIZE: 9pt; FONT-FAMILY: 宋体; mso-ascii-font-family: Verdana; mso-hansi-font-family: Verdana; mso-bidi-font-family: Tahoma; mso-font-kerning: 1.0pt ; mso-ansi-language: EN-US; mso-fareast-language: ZH-CN; mso-bidi-language: AR-SA”>Strong oxidants, strong acids, strong bases.
Storage method
Save in a dry and cool place
Synthesis method
Obtained from the amination of acrylonitrile. Cool the dry autoclave to 35℃ or below, add acrylonitrile, diphenylamine, and tert-butyl alcohol in sequence, stir 5min, Add liquid ammonia, control the temperature at 100-109℃, the pressure at about 1MPa, keep warm and stir 4h. Cool to below 10℃ and stop stirring when the pressure drops to normal pressure. Recover tert-butanol under reduced pressure at 65-70℃ (14.7-21.3kPa) to obtain crude product 3-Aminopropionitrile, the crude product is distilled under reduced pressure and collected 66-105℃ (1.33-4.0kPa) fraction to obtain 3- aminopropionitrile.
Purpose
Used as organic synthesis and pharmaceutical intermediates.
Intermediate for the synthesis of alanine and pantothenic acid; can be obtained by reduction1,3-Diaminopropane; reacts with phosgene to produce isocyanate, which can further react with p-nitroaniline to produce a sweetener that is sweeter than sugar350 times; the hydrogen on ammonia can be substituted by aliphatic hydrocarbons, alicyclic hydrocarbons, aromatic hydrocarbons and heterocyclic rings to generate Corresponding alkylaminopropionitrile;



 微信扫一扫打赏
微信扫一扫打赏
