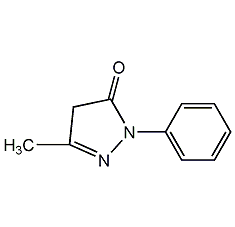
Structural formula
| Business number | 020J |
|---|---|
| Molecular formula | C10H10N2O |
| Molecular weight | 174.20 |
| label |
3-Methyl-1-phenyl-2-pyrazolin-5-one, 2,4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one, Edaravone, 1-Phenyl-3-methyl-5-pyrazolone, 2,4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one, C.I. Developer 1, Heterocyclic compounds |
Numbering system
CAS number:89-25-8
MDL number:MFCD00003138
EINECS number:201-891-0
RTECS number:UQ9625000
BRN number:609575
PubChem number:24885706
Physical property data
1. Appearance: white powder or crystal
2. Melting point (ºC): 127
3. Boiling point (ºC, 5.2kPa): 287
4. Refractive index: 1.637
5. Solubility: Solubility In hot water, alcohol, acid and alkali, slightly soluble in benzene, insoluble in ether and petroleum ether.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 51.20
2. Molar volume (cm3/mol): 148.3
3. Isotonic specific volume (90.2K ): 382.8
4. Surface tension (dyne/cm): 44.3
5. Polarizability (10-24cm3): 20.30
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 3
6. Topological molecule polar surface area 32.7
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 241
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Low toxicity, no reports of harm. Contact allergies were discovered during the production process. Inhaling its vapor will irritate the respiratory tract and cause chest tightness, cough, loss of appetite and other symptoms. Skin contact may cause redness and swelling. Once out of contact, general symptoms disappear and no lasting effects are seen.
Storage method
Packed in plastic bags, coated sacks or polypropylene woven bags or external wooden barrels, cardboard barrels, etc., each bag is 30kg, 40kg or 50g. Store in a dry and ventilated place. Protect from moisture and heat. Store and transport according to general chemical regulations��
Synthesis method
Using glacial acetic acid as raw material, pyrolysis at high temperature to obtain ketene, polymerization to obtain diketene, and then ammonolysis to obtain acetylacetamide.
In addition, aniline is used as raw material, diazotized with NaNO2 under acidic conditions (hydrochloric acid), and then ammonium bisulfite and ammonium sulfite are used as the The reducing agent is used for reduction, and sulfuric acid is added for hydrolysis to produce phenylhydrazine. Phenylhydrazine is condensed with acetyl acetamide to produce the product
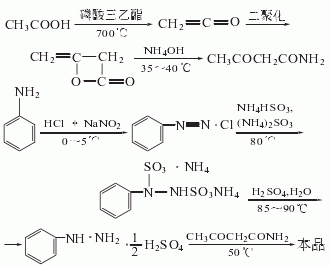
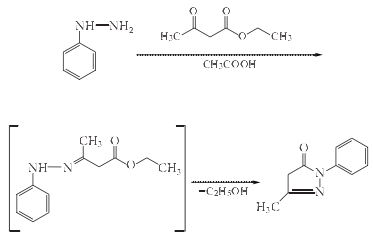
1. 2,4-Dihydro-5-methyl-2-phenyl-3H-pyrazole -Synthesis of 3-one (edaravone)
Add 108g (1mol) of phenylhydrazine, 130g (127.5ml, 1mol) of ethyl acetoacetate and 440ml of glacial acetic acid into the reaction bottle, and complete the addition. Stir and heat to flow temperature, stir and reflux for 3 to 4 hours. Use TLC to track [developing agent: petroleum ether/ethyl acetate (4:1)], and develop on a GF254 silica gel plate. The phenylhydrazine spots disappear and the reaction reaches the end point. Cool to room temperature, move to a distillation bottle and use a rotary evaporator to concentrate under reduced pressure to evaporate the solvent. The evaporated solvent is recovered and the remaining oil is cooled to room temperature. Add an appropriate amount of ethyl acetate, stir well and place it in the refrigerator overnight. Filter. The filter cake is washed with cold ethyl acetate or petroleum ether and drained to obtain 157g of crude 2,4-dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one (edaravone). , the yield is 90%, and the crude product is light yellow needle crystal.
2. Refining of Edaravone
Add 157g of crude product and 25%~30% ethanol into the reaction bottle Aqueous solution 420ml, stir and heat to reflux until completely dissolved, cool slightly. Add 25g of activated carbon, stir and reflux for 25 minutes. Filter while hot, drain, concentrate the filtrate under reduced pressure until a small amount of crystals precipitate on the wall of the distillation bottle, stop concentrating, and cool to 5~10ºC Crystallize and filter, and the filter cake is vacuum-dried at 50ºC to obtain 135g of white needle-shaped crystalline edaravone refined product, with a purification yield of 86% and a total yield of 77.4%, mp127~129ºC. Purity >99.5% (HPLC method ).
Purpose
1. Mainly used in the production of pharmaceutical antipyrine, aminopyrine, and metamizole. It is also used in dyes and color film dyes, pesticides and organic synthesis industries. middle. It can also be used as a chemical reagent for detecting vitamin B12, CO, Fe, Cu, Ni, etc.
2.This product is a new drug for the treatment of cerebrovascular disease. It is used to relieve acute Nervous system symptoms and impairment in daily life activities caused by cerebral infarction. The mechanism of action of this product is different from existing cerebral ischemia treatment drugs. It is a free radical scavenger that can effectively avoid cerebral ischemia. It protects brain tissue against brain damage. Foreign clinical studies have shown that if this product is used within 72h and 24h of onset, The effective rates for improving neurological symptoms and impairment in activities of daily living were 65% and 74% respectively, while the placebo group 32% and 25% respectively. They are clinically used to treat neurological damage caused by cerebral infarction, reduce disability, Promote recovery.
3. Used in the production of pyrazolone acid dyes such as acid medium red BN, acid medium red B, Orange GR, weakly acidic bright yellow G, neutral date red D-BN, permanent yellow G, leather spray red GL, orange 2RL, brown 2RL/active dispersed bright yellow 3G, yellow brown M-3GR, etc.



 微信扫一扫打赏
微信扫一扫打赏
