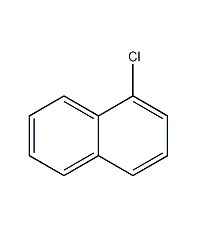
Structural formula
| Business number | 021Y |
|---|---|
| Molecular formula | C10H7Cl |
| Molecular weight | 162 |
| label |
1-Chloronaphthalene, α-Chloronaphthalene, α-Chloronaphthalene, α-Chloronaphthalene |
Numbering system
CAS number:90-13-1
MDL number:MFCD00003874
EINECS number:201-967-3
RTECS number:QJ2100000
BRN number:970836
PubChem number:24851177
Physical property data
1. Properties: The pure product is a colorless oily liquid. It is usually light yellow in color and has a creosote smell.
2. Boiling point (ºC, 101.3kPa): 260
3. Boiling point (ºC, 0.67kPa): 111~113
4. Melting point (ºC ): -2.3
5. Relative density (g/mL, 20/4ºC): 1.1938
6. Refractive index (n20ºC): 1.6326
7 . Viscosity (mPa·s, 25ºC): 2.940
8. Flash point (ºC, opening): 132
9. Fire point (ºC): above 558
10. Heat of evaporation (KJ/mol, b.p.): 52.08
11. Volume expansion coefficient (K-1): 0.000252
12. Solubility: Hardly soluble in water, soluble in ether, benzene, petroleum ether, alcohol and other organic solvents.
13. Relative density (25℃, 4℃): 1.1887
14. Solubility parameter (J·cm-3)0.5 : 21.161
15. van der Waals area (cm2·mol-1): 9.440×109
16. van der Waals volume (cm3·mol-1): 82.640
17. Gas phase Standard claimed heat (enthalpy) (kJ·mol-1): 119.8
18. Liquid phase standard claimed heat (enthalpy) (kJ·mol-1 sup>): 54.6
19. Liquid phase standard hot melt (J·mol-1·K-1): 242.7
Toxicological data
Poisoning through inhalation, ingestion or skin absorption can cause symptoms such as headache, vomiting, loss of appetite, conjunctivitis, hepatomegaly, and acute yellow liver atrophy in severe cases.
Ecological data
None
Molecular structure data
1. Molar refractive index: 48.99
2. Molar volume (cm3/mol): 135.5
3. Isotonic specific volume (90.2K ): 346.9
4. Surface tension (dyne/cm): 42.9
5. Polarizability (10-24cm3): 19.42
Compute chemical data
1. Hydrophobic parameter calculation parametersTest value (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Rotable Number of chemical bonds: 0
5. Number of tautomers: None
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 133
10. Number of isotope atoms: 0
11. Determine Number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determined number of chemical bond stereocenters: 0
14 .The number of uncertain chemical bond stereocenters: 0
15. The number of covalent bond units: 1
Properties and stability
α-Chloronaphthalene has low vapor pressure, high flash point, stable chemical properties, and does not corrode general metal materials. React with sodium hydroxide at 15°C above 300°C for 12 hours to generate α-naphthol. React with magnesium at 200~220℃ for several minutes to generate 10%~13% α-naphthyl magnesium chloride (α-C10H7MgCl). Nitrification is carried out at 0°C with a mixed acid of concentrated nitric acid and concentrated sulfuric acid. Nitro groups can be introduced into the 4-, 5-, and 8-positions of α-chloronaphthalene to generate three nitro derivatives of α-chloronaphthalene. Reacts with concentrated sulfuric acid at 140°C to generate 1-chloro-4-naphthalenesulfonic acid.
Storage method
This product should be stored in a sealed, cool, dry place. Can be stored in galvanized mild steel containers.
Synthesis method
Obtained from the direct chlorination of naphthalene: the chlorination reaction uses zinc as the catalyst, the naphthalene-chlorine ratio is 1:0.84-1.17, the catalyst dosage is 0.4-0.5% of the naphthalene weight, the chlorine flow time is 3-6h, and the reaction temperature is 90 -95℃, the average yield of 1-chloronaphthalene is 81.7%. After secondary fractionation, the product purity is 96%. Iodine can also be used as a catalyst to react in the solvent chlorobenzene. The reaction temperature is 60-70°C. The reaction is completed when chlorine is passed for 6 hours. The direct chlorination method simultaneously generates 2-chloronaphthalene, accounting for about 2-20%. The two isomers have similar boiling points (2-chloronaphthalene boiling point is 264~266°C), and their chemical properties are relatively similar, making it difficult to separate. In small quantities, it can also be obtained by diazotization and replacement of menaphthylamine.
Purpose
It is used in organic synthesis to prepare 1-naphthol, and can also be used as a solvent and analytical reagent. Used as insulation materials for wires, additives for special lubricants, pesticides, greases, and solvents for DDT, etc.



 微信扫一扫打赏
微信扫一扫打赏
