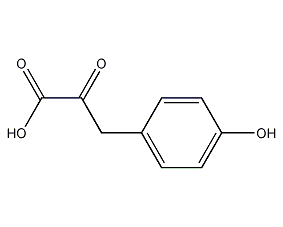
Structural formula
| Business number | 03ZL |
|---|---|
| Molecular formula | C10H8O4 |
| Molecular weight | 180.16 |
| label |
4-Hydroxyphenylpyruvate |
Numbering system
CAS number:156-39-8
MDL number:MFCD00002591
EINECS number:205-852-9
RTECS number:UZ1000000
BRN number:2691632
PubChem number:24847164
Physical property data
1. Properties: White or off-white flaky crystals.
2. Melting point (℃): 220℃ (decomposition)
3. Solubility: Soluble in ethanol, ether and ethyl acetate, slightly soluble in benzene and water.
Toxicological data
II. Toxicological data:
1. Acute toxicity: mouse LD50: 3,000 mg/kg; rat LD50: 3,250 mg/kg
Due to the LD50 is 3,000 mg/kg, and the acute toxicity of BPA is the same as that of table salt.
2. Teratogenicity
Mouse test: 1,600 mg/kg/day, increased maternal and fetal mortality and inhibited weight gain. There is no effect on the mother or fetus below 1,000 mg/kg/day.
Ecological data
None
Molecular structure data
1. Molar refractive index: 43.91
2. Molar volume (cm3/mol): 128.9
3. Isotonic specific volume (90.2K ): 365.5
4. Surface tension (dyne/cm): 64.5
5. Dielectric constant: not available
6. Dipole moment (10 -24cm3): Not available
7. Polarizability: 17.40
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 0.9
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 9
6. Topological molecule polar surface area 74.6
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 204
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Basic properties
Easily oxidized in alkaline solutions and irritating
Storage method
2. Storage
Should be sealed and stored at 4℃.
Synthesis method
1. Preparation method:
P-hydroxyphenyl aldehyde hydantoin (4): Add 6.11g (0.05 mol) of p-hydroxybenzaldehyde (2) and hydantoin (4) into a round-bottomed flask. 3) 5.5g (0.055mol), 10mL of dry piperidine, and install a reflux condenser (installed with a calcium chloride drying tube). The oil bath was slowly heated to 130°C and reacted for 30 minutes. Foam appears and boils. Cool, add200mL of water at ��60℃. Stir until clear liquid forms. Filter, and acidify the filtrate with about 20 mL of concentrated hydrochloric acid while cooling. Leave at room temperature for several hours. Filter the resulting yellow precipitate and wash with cold water. Dry in a vacuum dryer filled with potassium hydroxide to obtain crude product (4) 8.5-8.8, yield 83%-86%, mp 310-315°C. used directly for the next reaction. p-Hydroxyphenylpyruvate (1): In a reaction bottle equipped with a stirrer, reflux condenser, dropping funnel, and ventilation tube, add 8.5g (0.042mol) of compound (4), add high-purity nitrogen, and drip Add 240mL of 20% sodium hydroxide solution. Heat and boil in an oil bath at 170-180°C for 3 hours. Compound (4) dissolves quickly to form a clear orange solution, which becomes darker in color during the reaction. Cool the water bath, continue to flow nitrogen, add dropwise about 100 mL of concentrated hydrochloric acid to neutralize the alkali in it, control the dropping speed, and avoid forming a large amount of foam and obvious heat generation. Slowly add 5g of sodium bicarbonate to dissolve. Transfer the reaction solution to a continuous extractor and extract with diethyl ether until the diethyl ether layer is colorless. It takes about 2h. Separate the ether layer, carefully acidify the water layer with 60 mL of concentrated hydrochloric acid, then transfer it to an extractor and extract with ether until there is no more p-hydroxyphenylpyruvate in the ether layer. The ether layer was concentrated to dryness to obtain a slightly yellow solid crude product (1). Dry in a fast dryer filled with potassium hydroxide to obtain 6.9-7.2g of crude product (1), yield 92%-96%, mp 210-215°C (decomposition). Add 12 mL of water (83 to 86 mL) to each gram of crude product (1) and reflux in an oil bath at 150°C. After 10 to 20 minutes, a light yellow color will form and dissolve, then filter. Add 8.3 to 8.6 mL of concentrated hydrochloric acid (1.2 mL per gram of acid), and slowly cool to room temperature, stirring occasionally. After placing in the refrigerator for 10 to 20 minutes, a light yellow solution will form and filter. Add 8.3 to 8.6 mL of concentrated hydrochloric acid (1.2 mL per gram of acid), and slowly cool to room temperature, stirring occasionally. Place in the refrigerator for 10 hours, filter with suction, wash with cold water, and dry to obtain 4.4 to 4.7 g of compound (1), yield 59% to 63%, mp 216 to 218°C (decomposition). [1]
Purpose
3. Uses
Used for biochemical research. enzyme inhibitors.



 微信扫一扫打赏
微信扫一扫打赏
