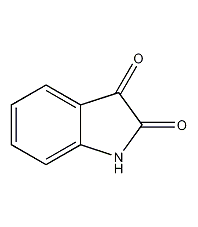
Structural formula
| Business number | 023N |
|---|---|
| Molecular formula | C8H5NO2 |
| Molecular weight | 147.13 |
| label |
Indole-2,3-dione, indigo, 1H-indole-2,3-dione, Isatin, Indoline-2,3-dione, fuel synthesis |
Numbering system
CAS number:91-56-5
MDL number:MFCD00005718
EINECS number:202-077-8
RTECS number:NL7873000
BRN number:383659
PubChem number:24881078
Physical property data
1. Properties: Orange-red single prism crystal.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 203.5℃ (partial sublimation)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2 kPa): Not determined
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation (º ): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Easily soluble in hot ethanol , slightly soluble in ether, soluble in hot water, benzene, acetone, soluble in alkali metal hydroxides.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 37.42
2. Molar volume (cm3/mol): 107.5
3. Isotonic specific volume (90.2K ): 291.0
4. Surface tension (dyne/cm): 53.6
5. Polarizability (10-24cm3): 14.83
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 6
6.Topological molecule polar surface area 46.2
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 212
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Number of uncertain atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. It turns purple when dissolved in alkali metal hydroxide, and turns yellow after standing. Reacts with amino acids and amines to produce various colors.
Storage method
Should be sealed and stored in a cool place.
Synthesis method
1. First, oximeacetanilide is prepared by condensation of aniline, trichloroacetaldehyde and oxime, and then cyclized and hydrolyzed to prepare isatin.
2. Mix and dissolve sodium carbonate, sodium nitrite, acetic acid, ice and water, then add sulfur dioxide gas under stirring until the ph value is 1. Control the temperature not to exceed 10℃ during the process. After the reaction is completed, steam is introduced to raise the temperature to above 30°C. Add trichloroacetaldehyde and anhydrous sodium carbonate to the obtained sodium hydroxylamine, stir thoroughly until dissolved, then add aniline, pass steam, heat to boil for 2 to 3 minutes, let stand for 1 to 1.5 hours, cool until crystallization is complete, and filter ,drying. The obtained isonitrosoacetanilide is added in batches to concentrated sulfuric acid at 50°C, and at the same time, stir thoroughly and indirectly cool with cold water, maintain the temperature between 60 and 75°C. After the addition of isonitrosoacetanilide is completed, raise the temperature to 80°C. After 10 to 15 minutes, pour the reaction solution into cold water and let it stand. After crystallization is complete, filter, wash and crystallize with water until neutral, and obtain a crude product. The crude product is precipitated by acid and alkali and recrystallized from ethanol to obtain the reagent 2,3-dione indene. The process reaction is:
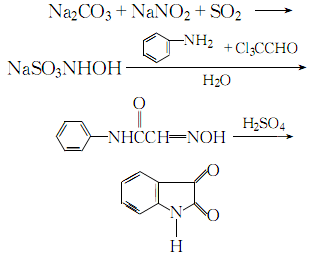
3. Preparation method:
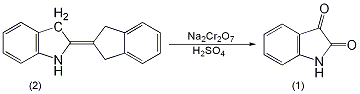
In a 2L reaction bottle equipped with a stirrer, thermometer and dropping funnel, add 130g (0.5mol) of indigo (2) and 400mL of water, and stir to form a uniform suspension. Add a solution of 175g (0.5mol) sodium dichromate dissolved in 200mL water, and then add 6.5g nitrobenzene. Cool in an ice water bath to about 5°C, add 285g of 63% sulfuric acid dropwise, control the reaction temperature between 0 and 10°C, and complete the addition in about 7 to 8 hours ①. Leave it overnight, then raise the temperature to 65°C and react for 1.5h. Cool to room temperature and filter. Discard the water layer (green), add the filter cake to 1300mL of water, heat to 50°C, and add sodium hydroxide aqueous solution until it becomes alkaline. Filter and recover 10g of unreacted indigo. After decolorizing the filtrate with activated carbon at 80°C, it was acidified with hydrochloric acid to pH 3-4, and bright orange crystals precipitated. Cool and stand for 24 hours, suction filter, wash with water until neutral, and dry at 100°C to obtain indigo (1) ② 105g, yield 77% (calculated based on the actual indigo participating in the reaction), mp 199 ~ 201°C . Note: ① During the addition of sulfuric acid, the reactant changes from blue to dark brown. After the addition of sulfuric acid, the reaction system becomes acidic. ② It can also be prepared by oxidizing indigo with dichromate in nitric acid. [1]
Purpose
1. This product is used as an intermediate for dyes and medicines, and is used in the production of the drugs Xincofen and Dye Disperse Yellow E-3G; in chemical analysis, it is a reagent for the determination of cuprous ions, thiols, thiophenes, and urinol. .
2.It is used as a reagent for the determination of amino acids and amines by thin layer analysis or low chromatography. It is also used for the determination of cuprous, thiols, thiophene, and for dye synthesis.



 微信扫一扫打赏
微信扫一扫打赏
