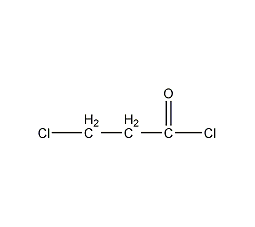
Structural formula
| Business number | 06RY |
|---|---|
| Molecular formula | C3H4Cl2O |
| Molecular weight | 126.97 |
| label |
3-Chloropropionyl chloride, β-Chloropropionyl chloride, β-Chloropropionly chloride, ClCH2CH2COCl |
Numbering system
CAS number:625-36-5
MDL number:MFCD00000747
EINECS number:210-890-4
RTECS number:None
BRN number:635814
PubChem number:24862061
Physical property data
1. Properties: colorless liquid.
2. Density (g/mL, 25/4℃): 1.325
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -32
5. Boiling point (ºC, normal pressure): 143-145
6. Boiling point (ºC, 5.2kPa): Not available Determined
7. Refractive index: 1.457
8. Flash point (ºC): 59
9. Specific rotation (º): Undetermined
p>
10. Autoignition point or ignition temperature (ºC): 460
11. Vapor pressure (kPa, 25ºC): 10
12. Saturated vapor pressure (kPa , 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15 . Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V ): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Slightly soluble in water.
Toxicological data
None yet
Ecological data
Do not allow large quantities of products that are slightly harmful to water to come into contact with groundwater, waterways or sewage systems. Do not discharge materials into the surrounding environment without government permission.
Molecular structure data
1. Molar refractive index: 25.67
2. Molar volume (cm3/mol): 97.9
3. Isotonic specific volume (90.2K ): 233.4
4. Surface tension (dyne/cm): 32.2
5. Polarizability (10-24cm3):10.17
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 17.1
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 52.8
10. Number of isotope atoms: 0
11. Number of determined atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
Keep away from oxides, moisture, alkalis, and reactive metals.
Storage method
Store in a container filled with dry inert gas and placed in a cool, dry place. The storage area must be locked and the keys must be given to the technical experts and their assistants. Avoid moisture and moisture. Keep away from oxidants, water sources, and do not store together with strong alkalis.
Synthesis method
1. Obtained from the reaction of propionic acid with sulfuryl chloride and thionyl chloride. Mix 59.2g propionic acid, 54.0g sulfuryl chloride and 61.6g carbon tetrachloride, then add 0.5g benzoyl peroxide. Reflux in the dark for 1.5 hours until no more gas escapes. Then add 190.4g of excess thionyl chloride and reflux for 4 hours. The solvent, excess thionyl chloride and propionyl chloride were then evaporated under normal pressure. Fractionate the residue under reduced pressure, collect the 51-54°C (13.3kPa) fraction into 2-chloropropionyl chloride, and collect the 81-84°C (13.3kPa) fraction into 3-chloropropionyl chloride, accounting for 45% and 55% respectively. , the total yield is 75%.
2. Preparation method:

In a reaction bottle equipped with a stirrer and a reflux condenser (the top is connected to a calcium chloride drying tube and connected to an acid gas absorption device), add 59.2g (0.8mol) of propionic acid (2) and sulfuryl chloride 54g, carbon tetrachloride 45mL, stir evenly and then add 0.5g benzoyl peroxide. Heat the reflux reaction in the dark for about 1.5 hours until no gas escapes. Then add 190g of excess thionyl chloride and reflux for 4 hours. Distill under normal pressure to evaporate the solvent, thionyl chloride and propionyl chloride. The residue is fractionated under reduced pressure. Collect the 51-54°C/13.3kPa fraction, which is 2-chloropropionyl chloride. Collect the 81-84°C/13.3kPa fraction. It is 3-chloropropionyl chloride (1). The two accounted for 45% and 55% respectively, and the total yield was 75%. [1]
Purpose
Used in organic synthesis.



 微信扫一扫打赏
微信扫一扫打赏
